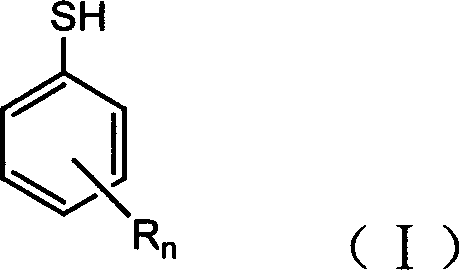
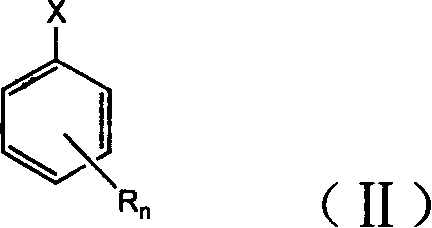
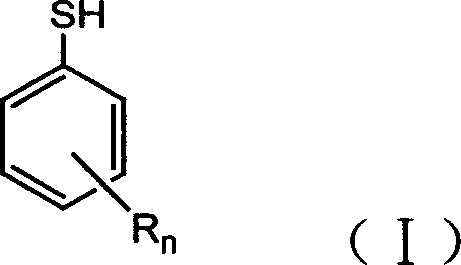
Preparation method of substituted thiophenol
A technology of thiophenol and halogenated benzene, which is applied in the field of preparation of substituted thiophenols, can solve the problems of high reaction conditions, high production costs, and high requirements for reaction conditions, and achieve simple preparation process, low environmental pollution, and high product yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Preparation of p-trifluoromethylthiophenol
[0021] 18 grams (0.1 moles) of p-trifluoromethylchlorobenzene and 28 grams (0.5 moles) of industrial sodium hydrosulfide pulverized are placed in a 500 milliliter three-necked flask, and 150 grams of solvent N, N-dimethylacetamide are added , heated and stirred, and reacted at 120° C. for 15 hours. After the reaction was completed, it was cooled, and dilute sulfuric acid was gradually added under stirring to acidify to pH=3. After steam distillation, 14.6 g of slightly light yellow transparent liquid was distilled out, with a yield of 82%. Boiling range 64~66°C (13 mm Hg), purity ≥98%, MS (m / z): 178 (M + ).
Embodiment 2
[0022] Embodiment 2 Preparation of p-nitrothiophenol
[0023] 15.8 grams (0.1 mole) of p-nitrochlorobenzene and 56 grams (1.0 mole) of industrial product sodium hydrosulfide of pulverization are placed in a 500 milliliter three-necked flask, and 150 grams of solvent N, N-dimethylformamide are added, heated Stir and react at 100°C for 10 hours. After the reaction was completed, it was cooled, and dilute sulfuric acid was gradually added under stirring to acidify to pH=3. Steam distillation yielded 13.9 g of light yellow solid with a yield of 90%. Melting point 77~81℃, purity ≥98%, MS(m / z): 155(M + ).
Embodiment 3
[0024] Embodiment 3 3, the preparation of 5-bis (trifluoromethyl) thiophenol
[0025] Put 24.9 grams (0.1 mole) of 3,5-bis(trifluoromethyl)chlorobenzene and 28 grams (0.5 mole) of industrial sodium hydrosulfide into a 500-milliliter three-necked flask, and add the solvent dimethyl sulfoxide 180 grams, heated and stirred, and reacted at 110°C for 10 hours. After the reaction was completed, it was cooled, and dilute sulfuric acid was gradually added under stirring to acidify to pH=4. After steam distillation, 19.7 g of slightly light yellow transparent liquid was distilled out, with a yield of 80%. Boiling point 63~65℃(15mm Hg), purity ≥98%, MS(m / z): 246(M + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com