Method for preparing baloxavir intermediate and intermediate obtained by method
A technology for intermediates and compounds, applied in the field of pharmaceutical synthesis, can solve the problems of unsuitability for industrial production, irritating odor of thiophenol, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Compound A-1
[0044]
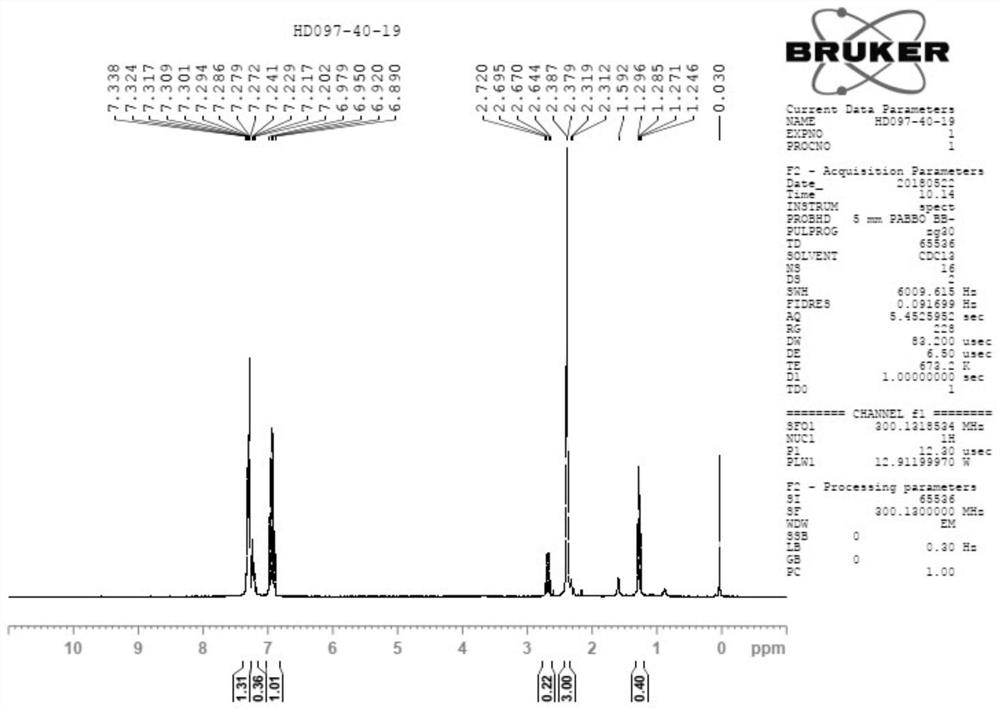
[0045] Add 3,4-difluorobromobenzene (20g) and tetrahydrofuran (40ml) into the reaction flask, under the protection of nitrogen, control the internal temperature below -50°C, drop lithium diisopropylamide (LDA, 62ml, 2mol / L tetrahydrofuran solution), the dropwise addition is completed, the internal temperature is controlled below -50°C, and the reaction is carried out for 1 hour, then the temperature is controlled below -20°C, and dimethyl sulfate (15.6g) is added dropwise, after the dropwise addition is completed, the temperature is naturally raised and stirred React for 1 to 2 hours. After the reaction was completed by the central control monitoring, post-processing steps such as quenching and extraction were carried out, and most of the solvent was removed by rotary evaporation to obtain the crude product of compound A-1 (containing part of the solvent). The H NMR spectrum of compound A-1 is shown in figure 1 .
[0046] Synt...
Embodiment 2
[0062]
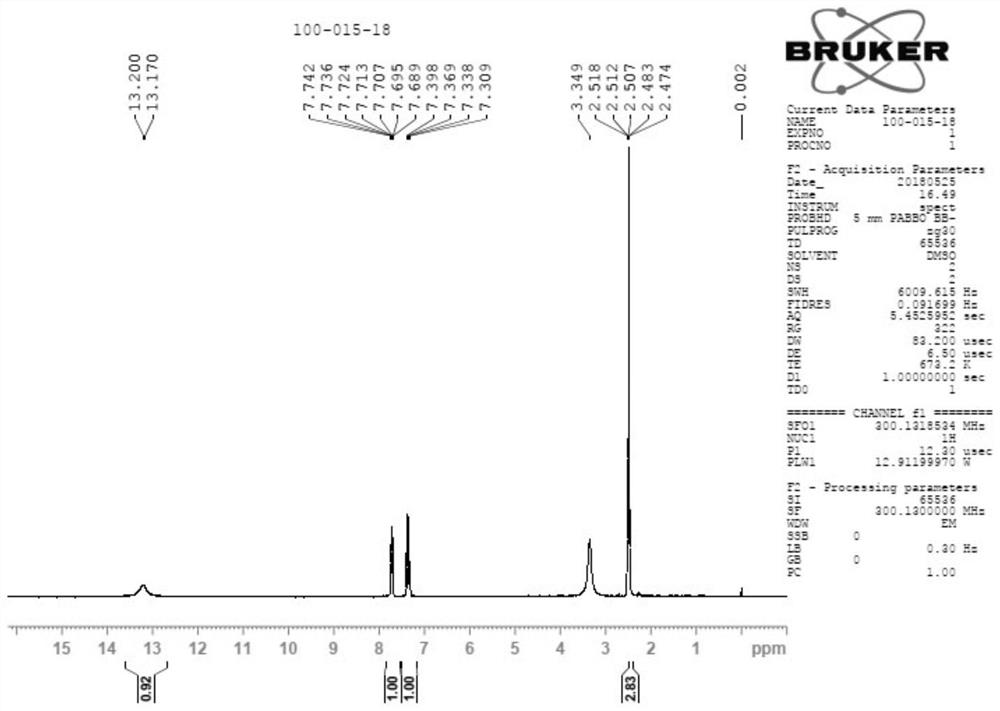
[0063] Under nitrogen protection, the temperature was controlled below -70°C, dry methyl tert-butyl ether and LDA (40mmol) were added to the reaction flask, the temperature was controlled below -60°C, and the raw material (30mmol) of formazan was added dropwise. Base tert-butyl ether solution; keep the temperature at -40°C, add iodomethane (42mmol), the reaction system immediately produces a yellow solid, and the reaction is exothermic violently. After the addition, the temperature of the system was raised to room temperature, and the reaction solution turned yellow with a large amount of solids. The reaction was carried out at room temperature for 3 hours, and the reaction was stopped by monitoring in the central control system. After post-processing, 13.5 g of the crude product of A-1 was obtained.
[0064] Synthesis of Compound A-2
[0065]
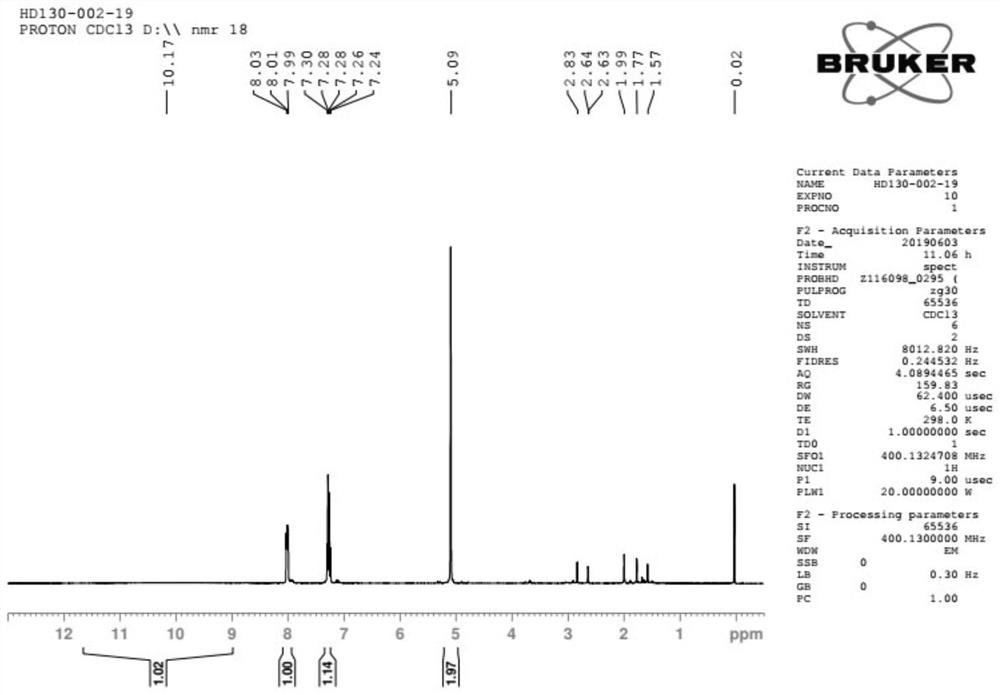
[0066] Add compound A-1 (482g) and tetrahydrofuran (250ml) into the reaction flask, protect it with nitrogen, control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com