Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38results about "Organic free radical generation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
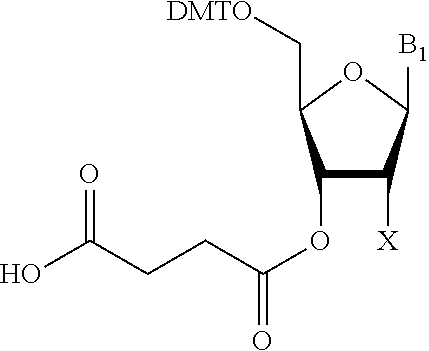
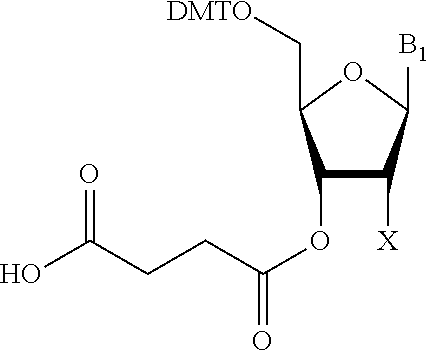
Solid-phase support for oligonucleotide synthesis and oligonucleotide synthesis method
ActiveUS9045573B2A large amountReduce loadSugar derivativesOrganic free radical generationPolyethylene glycolOligonucleotide synthesis
The present invention provides a solid-phase support for oligonucleotide synthesis for synthesizing long chain oligonucleotide, RNA oligonucleotide and modified oligonucleotide at high synthetic quantity and high purity with a low loading amount of a linker. Provided is a solid-phase support for oligonucleotide synthesis comprising a porous resin bead having a monovinyl monomer unit, a crosslinkable vinyl monomer unit and a polyethylene glycol unit and a cleavable linker loaded on its surface,the porous resin bead having a group capable of binding to a carboxy group by a dehydration condensation reaction on its surface, the cleavable linker having a carboxy group, wherein the carboxy group of the cleavable linker is bound to the group capable of binding to a carboxy group, by a dehydration condensation reaction, anda loading amount of the cleavable linker is 1 to 80 μmol / g relative to the weight of the porous resin bead.
Owner:NITTO DENKO CORP
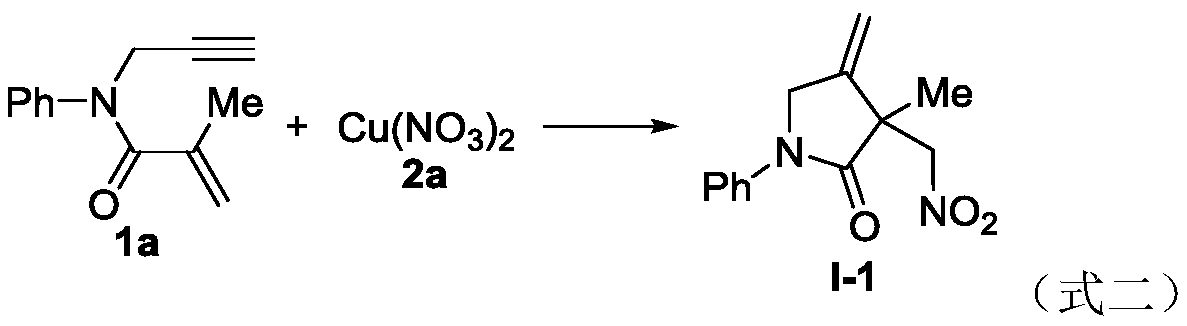
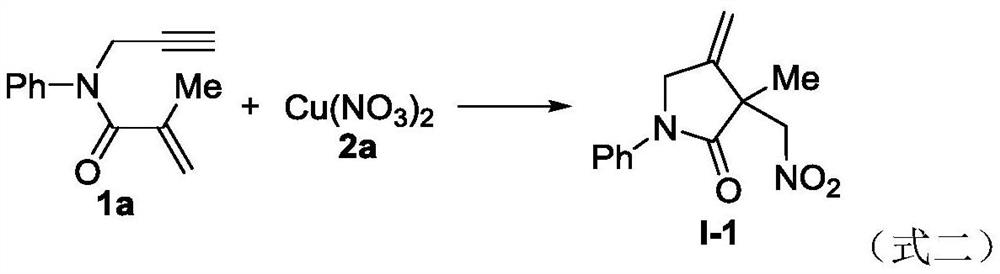
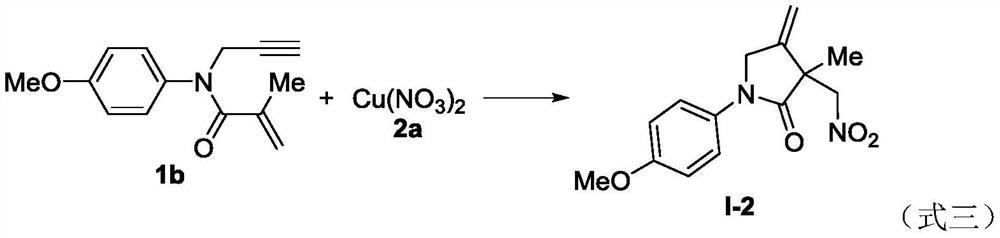
Novel method based on nitration/cyclization reaction of 1,6-eneyne compound
ActiveCN110467553AImprove responseExtensive hydrogen sourceOrganic free radical generationHydrogen2-Pyrrolidone
The invention relates to a novel method based on a nitration / cyclization reaction of a 1,6-eneyne compound. According to the method, the 1,6-eneyne compound and copper nitrate are used as raw materials and subjected to the nitration / cyclization reaction in a solvent under the action of an oxidizing agent until the raw materials are completely reacted, and then post-treatment is performed to obtaina nitro-substituted 2-pyrrolidone compound; in the process of the nitration reaction, participation of water in the solvent is not needed, and an intermediate with strong acidity such as nitrous acidis not produced, so reaction process is greatly simplified and is carried out under a neutral condition, the nitration reaction process has wide hydrogen sources, and high-regioselectivity and high-yield preparation of the nitro-substituted product is realized in a mild oxidation system. The method has the advantages of simple and convenient operation, usage of cheap and easily available raw materials, low cost, a wide application range of reaction substrates, mild reaction conditions, high efficiency and stability, is particularly suitable for industrial production, presents good applicationprospects, and provides a new thought for free radical nitration reactions.
Owner:YANGTZE NORMAL UNIVERSITY
Green synthesis method of amino alcohol compounds under visible light catalysis
ActiveCN110550992AThe synthetic route is simpleSimple and fast operationOrganic compound preparationOrganic free radical generationSynthesis methodsOrganic synthesis
The invention discloses a green synthesis method of amino alcohol compounds under visible light catalysis, and belongs to the technical field of organic synthesis. The green synthesis method specifically comprises the following steps that S1, 90.4mg of N-phenylglycine, 1.9mg of a photocatalyst, 2ml of water and 20.4ul of benzaldehyde are added in sequence into 10ml of a dried Schlenk tube; S2, three times of air extraction operation is conducted on a reaction bottle to ensure that no water or oxygen exists in a reaction tube before sealing; S3, the reaction bottle is placed under Blue LED irradiation, mixing is conducted until TLC detecting reaction is finished; and S4, 5 ml of ethyl acetate is correspondingly extracted for 4 times, organic phases are collected, anhydrous Na2SO4 is used for drying, appropriate silica gel is added and concentrated under decompression, and obtained residues are purified through column chromatography to obtain the product amino alcohol compounds. Comparedwith the prior art, the green synthesis method has the characteristics of simple synthesis route, easy and convenient operation, little environmental pollution, mild reaction conditions, good controllability of reaction conditions, and wide application range of substrates.
Owner:HANGZHOU NORMAL UNIVERSITY
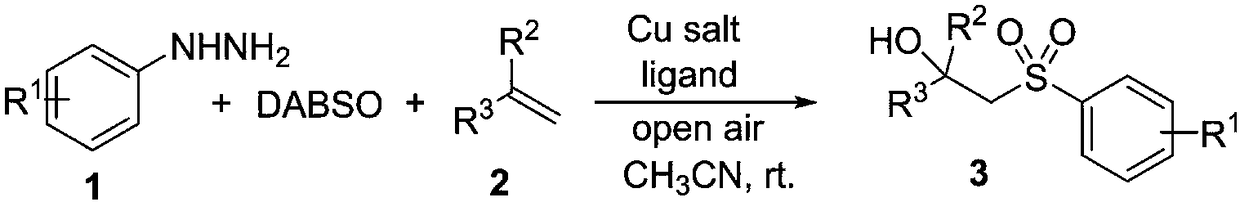
Synthesis method of beta-hydroxyl substituted sulfonyl compound
ActiveCN109134320AEasy to operateEfficient responseOrganic compound preparationOrganic free radical generationSynthesis methodsOxygen
The invention discloses a synthesis method of a beta-hydroxyl substituted sulfonyl compound. The synthesis method of the beta-hydroxyl substituted sulfonyl compound comprises the following steps: catalyzing reaction of phenylhydrazine and DABCO.(SO2)2 in the air by copper salt in an organic solvent and at room temperature to generate sulfonylfree radical so as to perform free radical addition on olefin; and then combining with oxygen molecules in the air to form a peroxide intermediate and performing catalytic reduction to prepare the beta-hydroxyl substituted sulfonyl compound. According to the method, the beta-hydroxyl substituted sulfonyl compound is constructed at one step by utilizing an olefin bifunctionalization strategy under very mild condition, and the functional group diversityof the sulfonyl compound is enriched; the reaction raw materials are simple and easily available; and the method is low in cost, simple to operate and suitable for large-scale preparation, and has good application prospect.
Owner:JIAXING UNIV
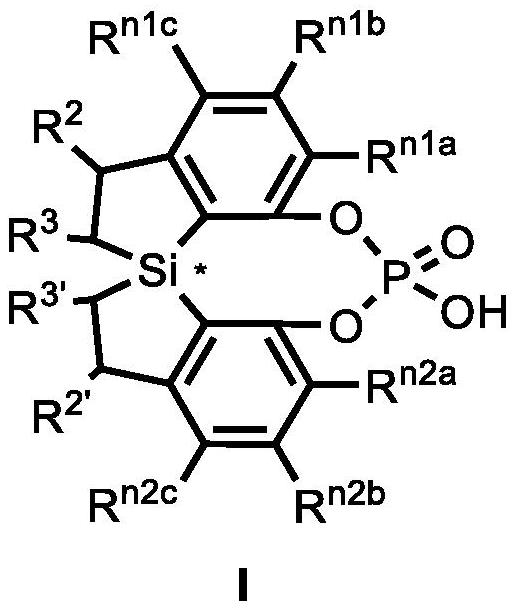
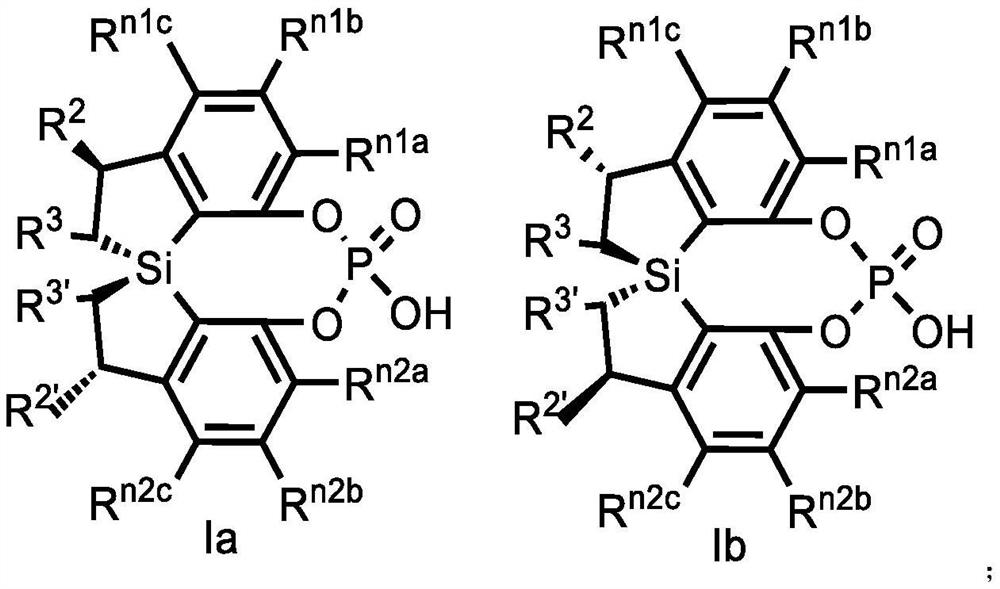
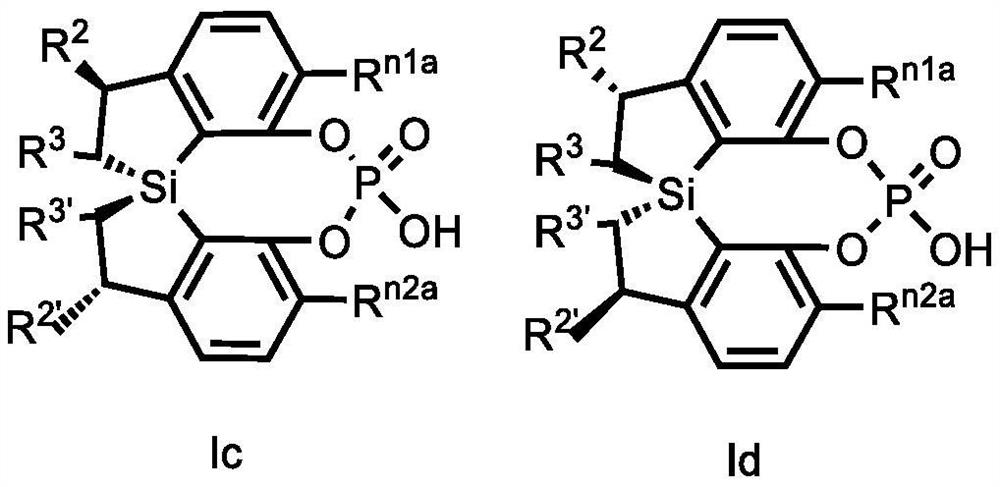
Spiro bis(dihydrobenzosilole) phosphoric acid compound as well as synthesis method and application of spiro bis(dihydrobenzosilole) phosphoric acid compound
ActiveCN113735908AHigh yieldSimple and fast operationOrganic compound preparationGroup 5/15 element organic compoundsO-Phosphoric AcidOrganic synthesis
The invention discloses a spiro bis(dihydrobenzosilole) phosphoric acid compound as well as a synthesis method and application of the spiro bis(dihydrobenzosilole) phosphoric acid compound. The invention provides a method for efficiently constructing a spiro bis(dihydrobenzosilole) phosphoric acid compound as shown in a formula I by using a spiro bis(dihydrobenzosilole) diphenol compound. The method has the characteristics of simplicity and convenience in operation, easiness in obtaining raw materials, mild reaction conditions, high yield, convenience in purification and the like. The spiro bis(dihydrobenzosilole) phosphoric acid compound as shown in the formula I provided by the invention can be directly used as a chiral organic catalyst, or can be used for generating metal complexes with metal salts from the third group to the thirteenth group or can be mixed in situ for use, and is used for catalyzing an asymmetric organic synthesis reaction.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Composite metal photocatalysis system and preparation method and application thereof
ActiveCN110975940AFast transferEfficient transferOrganic-compounds/hydrides/coordination-complexes catalystsOrganic free radical generationPtru catalystCycloaddition
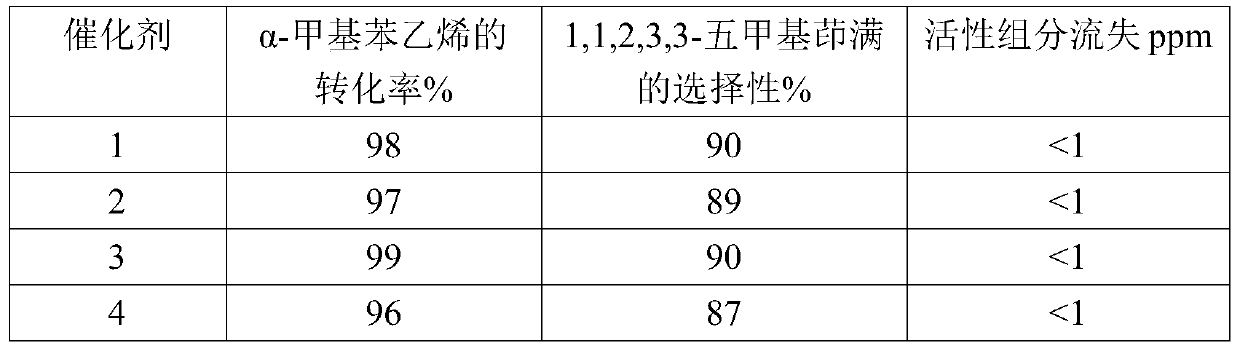
The invention discloses a composite metal photocatalysis system which is represented by Ni-M-X-Y-Z, wherein M is selected from the group consisting of Al, Ag, Bi, Cd, Ge, Sb and Sn; x is selected fromthe group consisting of triphenylphosphine, triethylphosphine, tributylphosphine, tri-tert-butylphosphine and tricyclohexylphosphine; Y is selected from the group consisting of carbon nanorods, molecular sieves, ordered mesoporous carbon and silicon dioxide; Z is selected from the group consisting of diphenyl disulfide, dicumyl peroxide, hydrazine and 2,4-dinitrophenylhydrazine; the mass fractionof Ni is 10-20%; the mass fraction of M is 20-30%; the mass fraction of X is 20-40%; the mass fraction of Y is 15-40%,; and the mass fraction of Z is 10-30%. The catalytic system is used for preparing 1,1,2,3,3-pentamethylindane through free radical cycloaddition of alpha-methylstyrene and 2-methyl-2-butene, an conversion rate is larger than 90%, and selectivity is larger than 85%; and a catalystis stable and not prone to loss in the free radical cycloaddition reaction. A method provided by the invention is easy to operate and good in economic benefit.
Owner:WANHUA CHEM GRP CO LTD
Radical generating catalyst, method for producing radical, method for producing oxidation reaction product, drug, and drug for agriculture and livestock
ActiveUS20180369798A1Mild conditionsImprove securityAntibacterial agentsPreparation by oxidation reactionsSimple Organic CompoundsLivestock
An object of a first aspect of the present invention is to provide a radical generating catalyst that can generate (produce) radicals under mild conditions. In order to achieve the above object, a first radical generating catalyst according to the first aspect of the present invention is characterized in that it includes ammonium and / or a salt thereof. A second radical generating catalyst according to the first aspect of the present invention is characterized in that it includes an organic compound having Lewis acidic properties and / or Brønsted acidic properties.
Owner:ACENET
Method for preparing aryl ketone based on iron-catalyzed free radical-free radical coupling reaction such as ketonic acid decarboxylation and fatty aldehyde de-carbonylation
ActiveCN111056890ALow costToxicOrganic compound preparationOrganic free radical generationPtru catalystKetonic acids
The invention discloses a method for preparing an aryl ketone derivative based on a free radical-free radical cross-coupling reaction such as ketonic acid decarboxylation and fatty aldehyde de-carbonylation. The method comprises the following steps: reacting aryl-substituted ketonic acid with fatty aldehyde under the catalytic action of ferric triacetylacetonate to generate an aryl ketone derivative; the gram-grade reaction can be realized by the method only by using 3mol% of an iron catalyst; and the method has the advantages of no need of consumption of a large amount of a Lewis acid catalyst or a stoichiometric organic metal reagent, mild reaction conditions, one-step reaction, few by-products, wide substrate application range and scalable reaction, and overcomes the defects of large catalyst consumption, insufficient functional group tolerance, many by-products and the like in the prior art.
Owner:XIANGTAN UNIV
Sulfonylation/cyclization reaction method of 1, 6-eneyne and sulfonyl chloride driven by visible light
ActiveCN111348980AMild reaction conditionsSuitable for industrial productionOrganic free radical generationSulfonyl chlorideLED lamp
The invention relates to a visible-light-driven sulfonylation / cyclization reaction method of a 1, 6-enyne compound and a sulfonyl chloride compound in the absence of a photocatalyst and an additive system. The method comprises the following steps: adding the 1, 6-enyne compound, the sulfonyl chloride compound and a solvent into a Schlenk reaction bottle, and carrying out a stirring reaction undera 3W blue LED lamp at a certain temperature under an air atmosphere condition to obtain a sulfonylation / cyclization product.
Owner:NINGBO UNIV
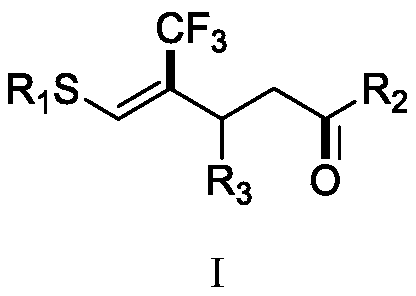
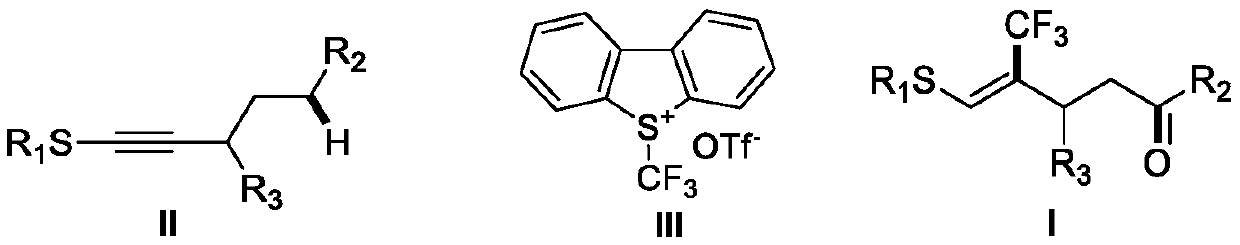
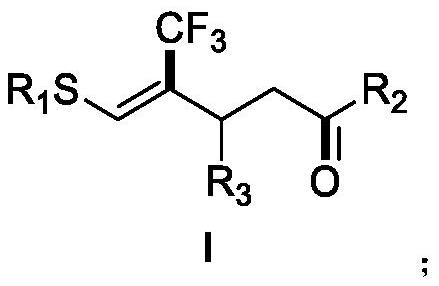
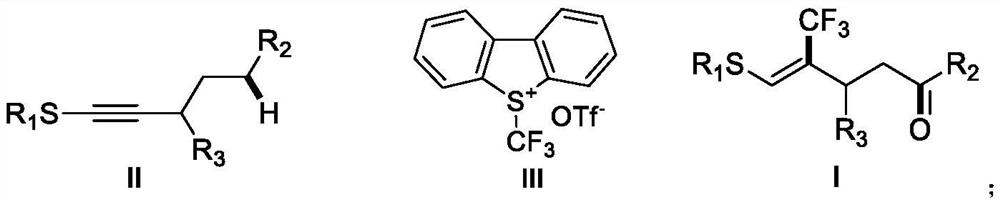
(Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative and preparation method therefor
ActiveCN110698313AAchieving a cascade reactionExpand areaGroup 4/14 element organic compoundsOrganic free radical generationThio-Alkyne
The invention discloses a (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative and a preparation method therefor. The method comprises the steps of adding and mixing alkyne thioether with a structure represented by a formula II, S-(trifluoromethyl)dibenzothiophene onium trifluoromethyl sulfonate with a structure represented by a formula III, ruthenium tri(2,2-bipyridyl)dichloride, alkali anda solvent, forming a reaction system under blue-light irradiation, and carrying out aftertreatment after a reaction is completed, thereby obtaining the (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative with a structure represented by a formula I. According to the method, the stereoselective synthesis of the (Z)-4-trifluoromethyl-5-thio-alkyl-4-pentenone derivative is achieved in one step.The reaction conditions are mild, the substrate applicable range is broad, the reaction yield is good, the operation is simple, and a new way is provided for synthesis of trifluoromethyl containing pentenone compounds.
Owner:ZHEJIANG NORMAL UNIVERSITY
Solid-phase support for oligonucleotide synthesis and oligonucleotide synthesis method
ActiveUS20130317206A1A large amountLow loading amountSugar derivativesOrganic free radical generationPolyethylene glycolOligonucleotide synthesis
The present invention provides a solid-phase support for oligonucleotide synthesis for synthesizing long chain oligonucleotide, RNA oligonucleotide and modified oligonucleotide at high synthetic quantity and high purity with a low loading amount of a linker. Provided is a solid-phase support for oligonucleotide synthesis comprising a porous resin bead having a monovinyl monomer unit, a crosslinkable vinyl monomer unit and a polyethylene glycol unit and a cleavable linker loaded on its surface, the porous resin bead having a group capable of binding to a carboxy group by a dehydration condensation reaction on its surface, the cleavable linker having a carboxy group, wherein the carboxy group of the cleavable linker is bound to the group capable of binding to a carboxy group, by a dehydration condensation reaction, and a loading amount of the cleavable linker is 1 to 80 μmol / g relative to the weight of the porous resin bead.
Owner:NITTO DENKO CORP
Preparation method of 2-(2, 2, 6, 6-tetramethyl piperidine nitroxide radical-4-subunit) acetic acid derivative and application thereof
InactiveCN112851571AEasy to removeStable in natureOrganic-compounds/hydrides/coordination-complexes catalystsOrganic free radical generationAcetic acidPtru catalyst
The invention discloses a preparation method of a 2-(2, 2, 6, 6-tetramethyl piperidine nitroxide radical-4-subunit) acetic acid derivative and an application thereof. According to the 2-(2, 2, 6, 6-tetramethylpiperidine nitroxide radical-4-subunit) acetic acid derivative, commercialized 2, 2, 6, 6-tetramethyl-4-piperidone is subjected to simple oxidation to obtain 4-carbonyl-2, 2, 6, 6-tetramethylpiperidine nitroxide radicals, then the 4-carbonyl-2, 2, 6, 6-tetramethylpiperidine nitroxide radicals react with corresponding phosphorylation reagents, hydrolysis is carried out, the raw materials used in the method are cheap and easy to obtain, compared with the 2, 2, 6, 6-tetramethylpiperidine nitroxide radicals in the production process, the production efficiency is high, and the production cost is low, the compound is stable in property, basically free of any smell, convenient to store and capable of being stored at the room temperature, meanwhile, the preparation method is simple and easy to obtain, and when the compound is used as a catalyst, due to the fact that the compound is high in activity, large in solubility in a water phase and extremely small in catalyst dosage.
Owner:陕西海辰风扬医药科技有限公司
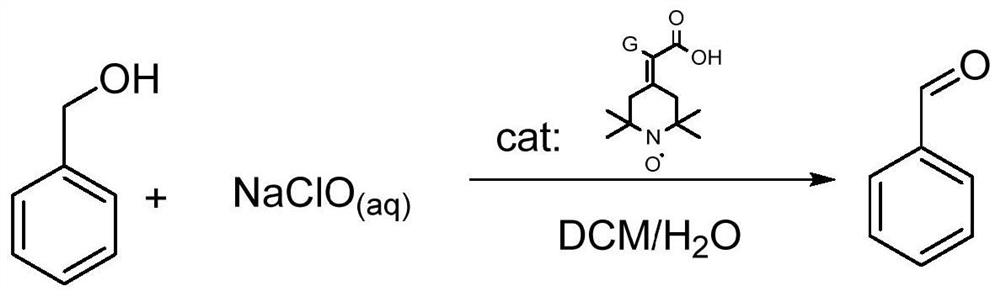
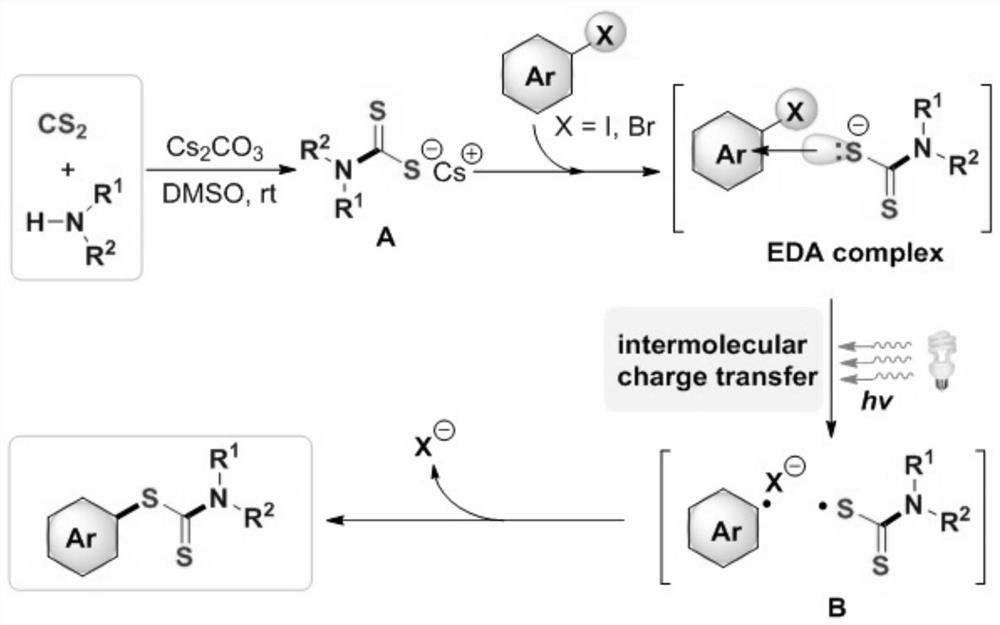
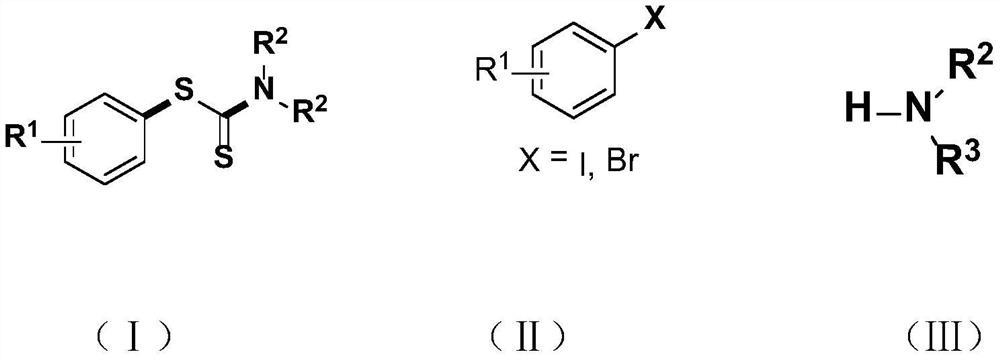
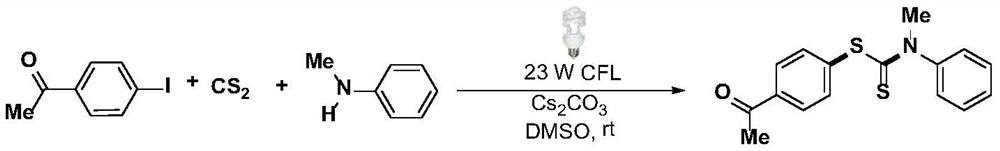
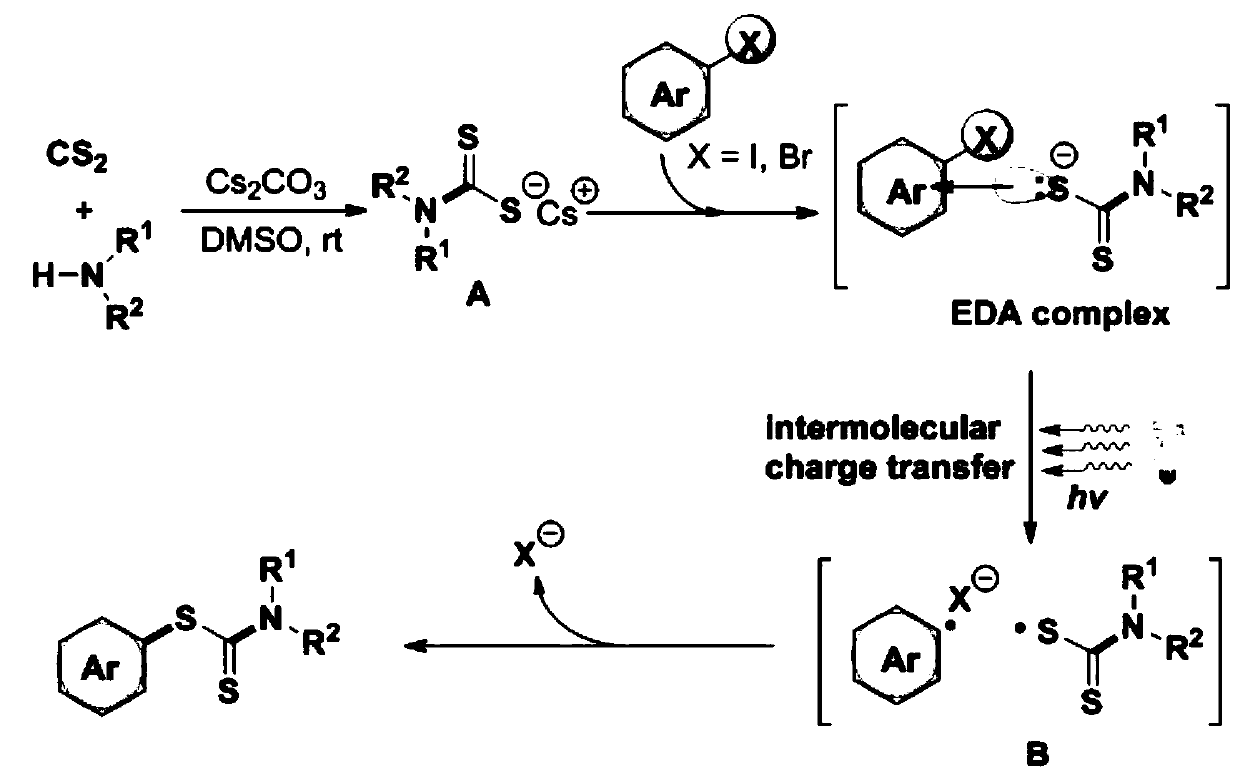
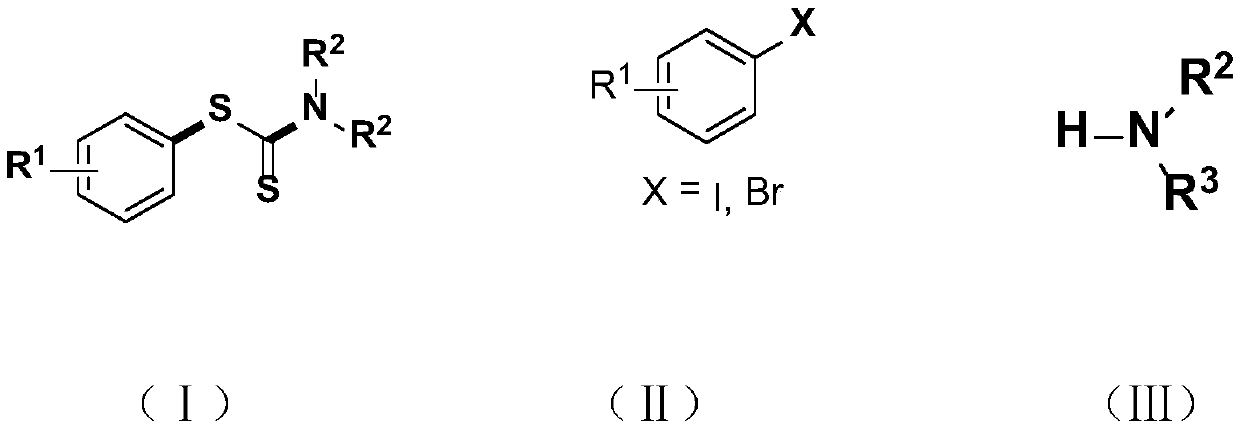
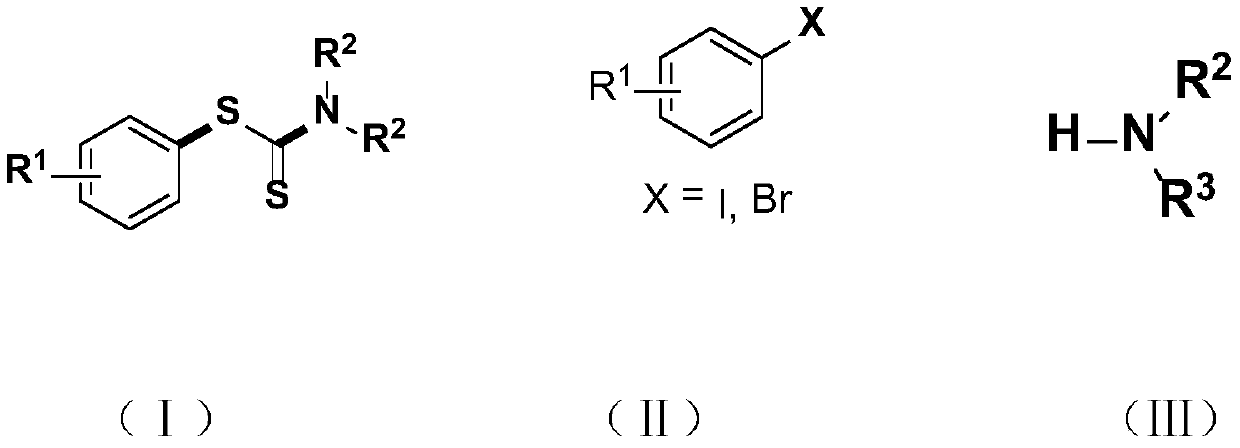
Synthesis method and application of a kind of visible light-promoted dithiocarbamate compound
ActiveCN110668987BImprove compatibilitySimple processAntibacterial agentsOrganic free radical generationCarbamateThio-
The invention relates to a method for synthesizing visible light-promoted dithiocarbamate compounds. The method uses substituted halogenated aromatic hydrocarbons, carbon disulfide and secondary amines as raw materials to construct dithiocarbamate compounds through a "one-pot method" under the irradiation of a 23-watt fluorescent lamp. The preparation method of the method is simple in process and device, can complete the reaction in one step, and has the advantages of few reaction steps, low cost, high yield, good functional group compatibility and the like.
Owner:QINGDAO UNIV OF SCI & TECH
A kind of (z)-4-trifluoromethyl-5-sulfanyl-4-pentenone derivative and its preparation method
ActiveCN110698313BAchieving a cascade reactionExpand areaGroup 4/14 element organic compoundsOrganic free radical generationRuthenium chlorideMethyl palmoxirate
Owner:ZHEJIANG NORMAL UNIVERSITY
A new method based on nitration/cyclization of 1,6-enynes
ActiveCN110467553BImprove responseExtensive hydrogen sourceOrganic free radical generationRegioselectivityCopper nitrate
Owner:YANGTZE NORMAL UNIVERSITY
Conjugated double-free-radical molecule as well as preparation method, spin distribution regulation and control method and application thereof
ActiveCN111807988AEasy to manufactureShort timeSolid-state devicesSemiconductor/solid-state device manufacturingCombinatorial chemistryOrganic chemistry
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Quadruple helicene radical molecules with thermally induced hysteresis loops and their preparation methods and applications
ActiveCN112778105BExpand and extend the π systemReduce energy level differenceOrganic compound preparationOrganic free radical generationMagnetic exchangeElectronic structure
The invention belongs to the field of organic magnetic materials, and specifically discloses a quadruple helicene free radical molecule characterized by thermally induced magnetic hysteresis loops of conjugated free radicals. The invention also discloses the preparation method of the quadruple helicene free radical, the thermally induced magnetic switching mechanism and the characteristics of the magnetic switching material. The invention constructs helicene multi-radical molecules, and the temperature can regulate the electronic structure of the free radical molecules and the magnetic exchange effect of spin electrons at the molecular level, so that it can show the change of material magnetism under macroscopic conditions. It can be applied to organic magnetic function conversion materials and organic spintronic devices.
Owner:HUNAN UNIV
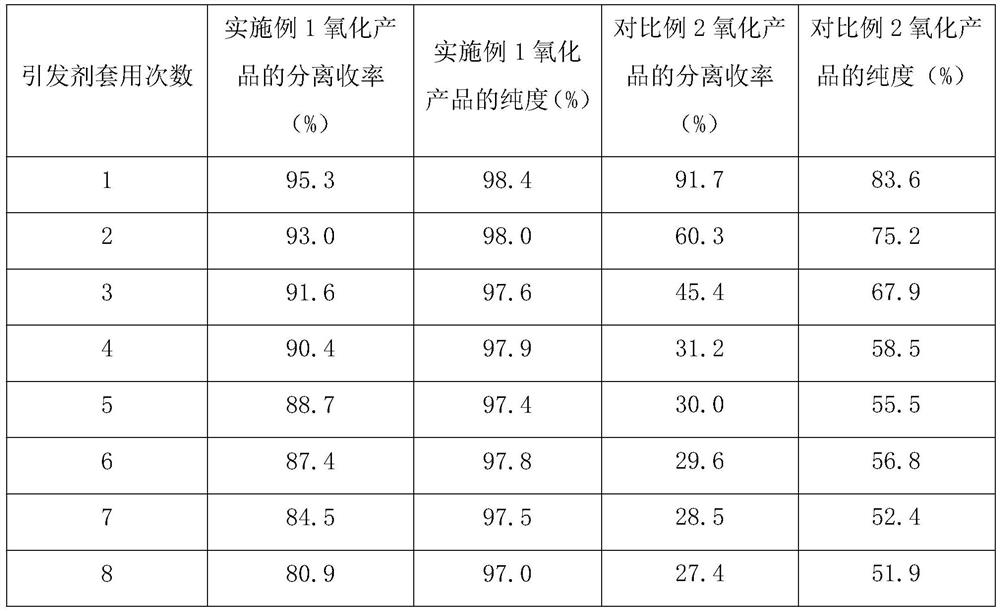
Preparation method of free radical initiator and application of free radical initiator in oxidation reaction
PendingCN113603626AHigh purityHigh yieldOrganic compound preparationOrganic free radical generationImideHydroxylamine
The invention discloses a preparation method of a free radical initiator, the preparation method comprises the following steps: feeding a hydroxylamine raw material, inorganic base and water according to a ratio, and carrying out acid-base neutralization reaction at 0-25 DEG C to dissociate hydroxylamine; adding tetrachlorophthalic anhydride, stirring at room temperature to react for 0.5-2 hours, reacting at 60-95 DEG C for 4-12 hours, washing with water, and drying at 60-80 DEG C for 10-24 hours to obtain an N-hydroxyl tetrachlorophthalimide crude product; and recrystallizing the N-hydroxyl tetrachlorophthalimide crude product in a mixed solvent of toluene and ethanol, filtering, and drying at 60-80 DEG C to obtain the N-hydroxyl tetrachlorophthalimide free radical initiator. According to the method, the adopted raw materials are simple, the conditions are easy to control, the preparation technology is green and environmentally friendly, the preparation repeatability is good, the free radical initiator is used in the oxidation reaction, a high-purity and high-yield product can be obtained, and the product obtained after repeated use still has high separation yield and purity.
Owner:WUHAN QIANGFENG XINTE TECH +1
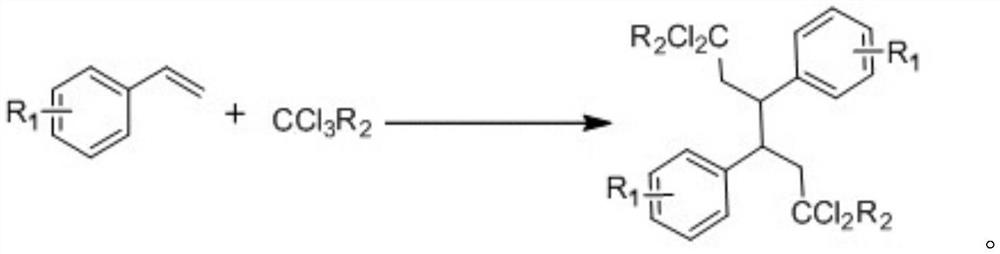
A kind of preparation method of halogenated alkanes synthesized with styrene and its derivatives and trichloroalkane
ActiveCN109096046BPlay a protective effectNot easily oxidizedCarboxylic acid nitrile preparationOrganic compound preparationAlkanePtru catalyst
The invention discloses a halogenated alkane synthesized by using styrene and its derivatives and trichloroalkane and a preparation method thereof. By using styrene and its derivatives and trichloroalkane as substrates, chloroalkyl is carried out under light conditions. and dimerization reaction, reacting 10-14h to prepare trifluorodichloroalkane; wherein the reaction temperature is 10-45°C, and the reaction is carried out under normal pressure simultaneously; As a catalyst, the reaction speed is accelerated; at the same time, alkali, iodine source, triphenylphosphine and deionized water are added to the whole reaction system; the whole reaction is carried out in a solvent; meanwhile, the whole reaction needs to be carried out under a nitrogen atmosphere. After the reaction is completed, the halogenated alkane can be obtained after purification treatment, and the halogenated alkane is a tail-to-tail dimerization product prepared by using styrene and its derivatives as reactants. At the same time, the invention has the advantages of simple and easy-to-obtain raw materials, novel and simple preparation process, less pollution, low energy consumption and high yield.
Owner:WENZHOU UNIVERSITY
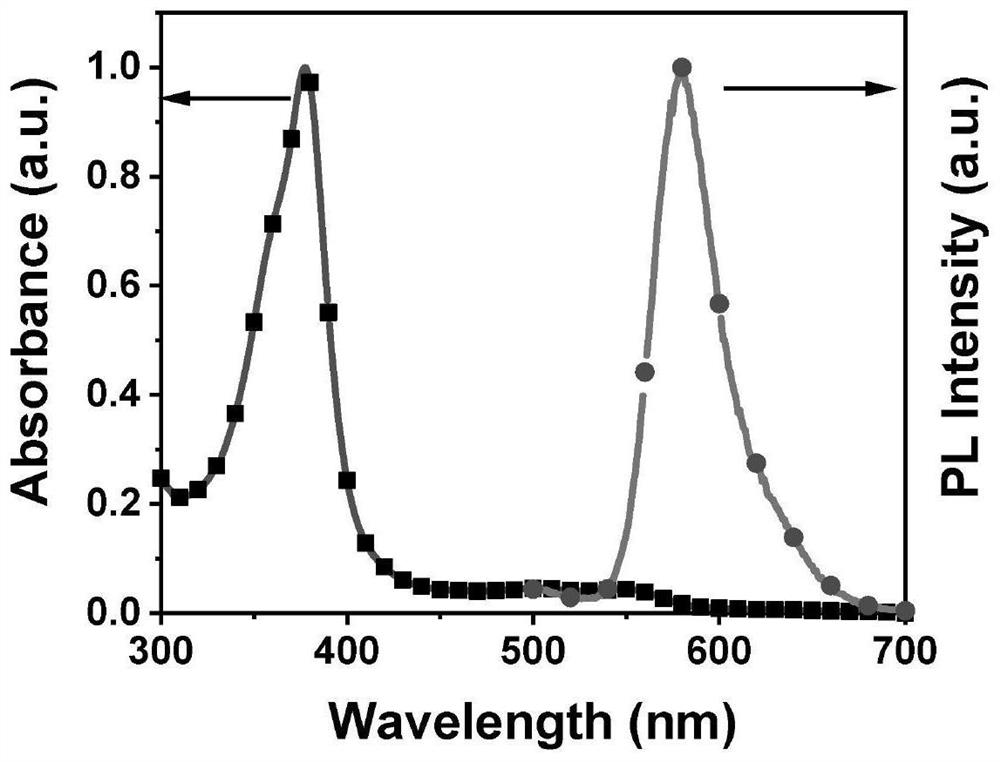
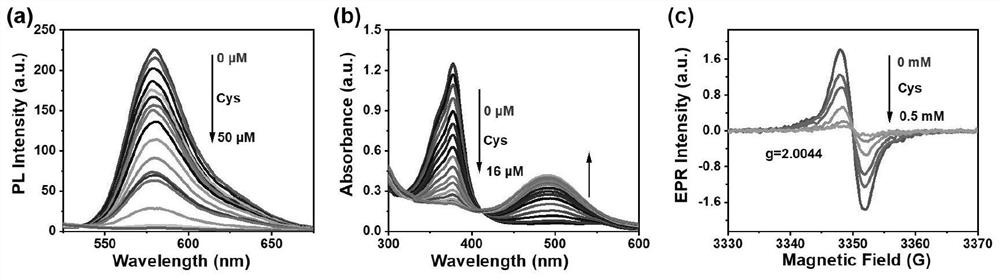
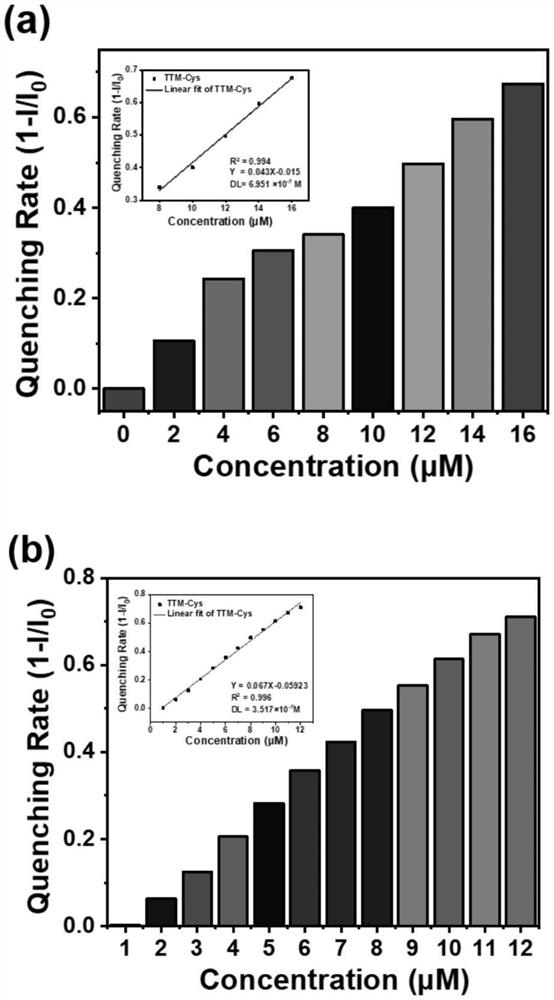
A trityl-based luminescent free radical material and its preparation method and application
ActiveCN113603598BTo achieve the detection purpose of multiple responsesRealize the detection purposeMaterial analysis by observing effect on chemical indicatorOrganic free radical generationAssayUltraviolet
The invention provides a trityl-based light-emitting free radical material and a preparation method and application thereof, belonging to the technical field of organic light-emitting materials. In the trityl radical luminescent material provided by the present invention, the common feature is the trityl radical connected to the nitro group with a strong electron-withdrawing effect; the central trityl radical not only acts as a The luminescent construction group also serves as an activation group to make trityl radicals more likely to be nucleophilicly substituted by biothiols. The synergistic effect of the two units (trityl radical and nitro group) enables the material to realize the detection purpose of multiple responses to biological thiols; to realize the detection of cysteine three channels (fluorescence, ultraviolet and EPR signals). According to the above strategies, the purpose of identifying biothiols in RGB patterning, simulated clinical assays and fluorescence imaging was achieved.
Owner:JILIN UNIV
Green Synthesis of Amino Alcohols Catalyzed by Visible Light
ActiveCN110550992BThe synthetic route is simpleSimple and fast operationOrganic compound preparationOrganic free radical generationOrganic synthesisBenzaldehyde
The invention discloses a green synthesis method of amino alcohol compounds under visible light catalysis, and belongs to the technical field of organic synthesis. It specifically includes the following steps: S1. Add 90.4mg N-phenylglycine, 1.9mg photocatalyst, 2ml water, and 20.4ul benzaldehyde to a dry 10ml Schlenk tube sequentially; S2. Perform three gas exchange operations on the reaction bottle to ensure Seal the reaction tube after it is free of water and oxygen; S3, place the reaction under the irradiation of Blue LED and stir until the reaction is detected by TLC; S4, extract 4 times with 5ml of ethyl acetate, collect the organic phase, and wash with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the resulting residue is purified by column chromatography to obtain the product aminoalcohol compound. Compared with the prior art, the method has the characteristics of simple synthetic route, convenient operation, less environmental pollution, mild reaction conditions, good controllability of reaction conditions, wide application range of substrates and the like.
Owner:HANGZHOU NORMAL UNIVERSITY
Visible light promoted synthesis method and application of dithiocarbamate compounds
ActiveCN110668987AImprove compatibilitySimple processAntibacterial agentsOrganic free radical generationCarbon disulfideThio-
The invention relates to a visible light-promoted synthesis method of dithiocarbamate compounds. The method uses substituted halogenated aromatic hydrocarbon, carbon disulfide and secondary amine as raw materials to construct the dithiocarbamate compounds through a one-pot method under the irradiation of a 23-watt fluorescent lamp. The preparation method has simple process and device, can completethe reaction in one step, and has the advantages of fewer reaction steps, low cost, high yield, good functional group compatibility and the like.
Owner:QINGDAO UNIV OF SCI & TECH
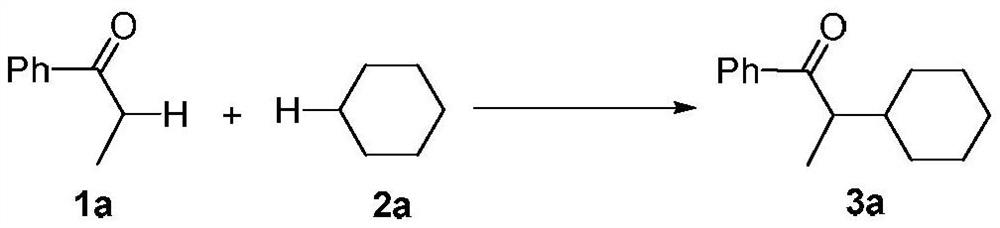
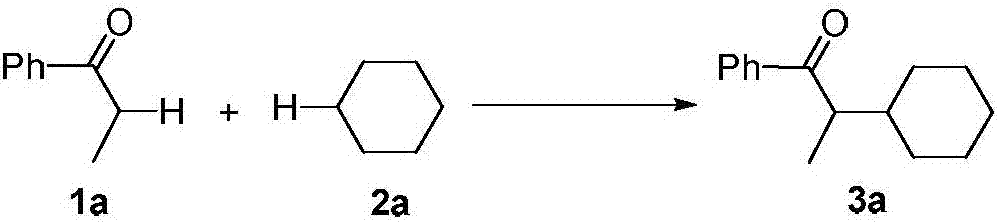
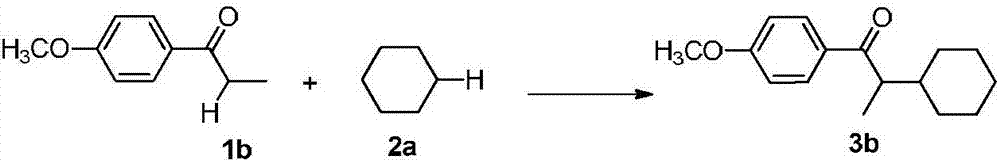
A kind of synthetic method of α-position cycloalkyl substituted ketone compound
InactiveCN107141209BAvoid emissionsAtom economy is highOrganic compound preparationPreparation by dehydrogenationOrganic synthesisDehydrogenation
The invention discloses a synthetic method for an Alpha-bit naphthenic-substituted ketone compound, and belongs to the technical field of the organic synthesis. The technical solution of the synthetic method comprises the following steps: mixing a ketone compound and naphthenic hydrocarbon or dissolving in an organic solvent, reacting in the existence of microwave radiation and a radical initiator in 140-170 DEG C of the temperature, to obtain a target product of the Alpha-bit naphthenic-substituted ketone compound. According to the synthetic method, the ketone compound and the simple naphthenic hydrocarbon are used as the raw materials, and the Alpha-bit naphthenic-substituted ketone compound is obtained by a cross dehydrogenation coupled reaction. The method has the advantages of cheap and simple raw materials, short reaction time, no metal catalysis, environmental protection and high atom economy, and is suitable for the industrial production.
Owner:HENAN NORMAL UNIV
Synthetic method for Alpha-bit naphthenic-substituted ketone compound
InactiveCN107141209AAvoid emissionsAtom economy is highOrganic compound preparationPreparation by dehydrogenationOrganic synthesisDehydrogenation
The invention discloses a synthetic method for an Alpha-bit naphthenic-substituted ketone compound, and belongs to the technical field of the organic synthesis. The technical solution of the synthetic method comprises the following steps: mixing a ketone compound and naphthenic hydrocarbon or dissolving in an organic solvent, reacting in the existence of microwave radiation and a radical initiator in 140-170 DEG C of the temperature, to obtain a target product of the Alpha-bit naphthenic-substituted ketone compound. According to the synthetic method, the ketone compound and the simple naphthenic hydrocarbon are used as the raw materials, and the Alpha-bit naphthenic-substituted ketone compound is obtained by a cross dehydrogenation coupled reaction. The method has the advantages of cheap and simple raw materials, short reaction time, no metal catalysis, environmental protection and high atom economy, and is suitable for the industrial production.
Owner:HENAN NORMAL UNIV
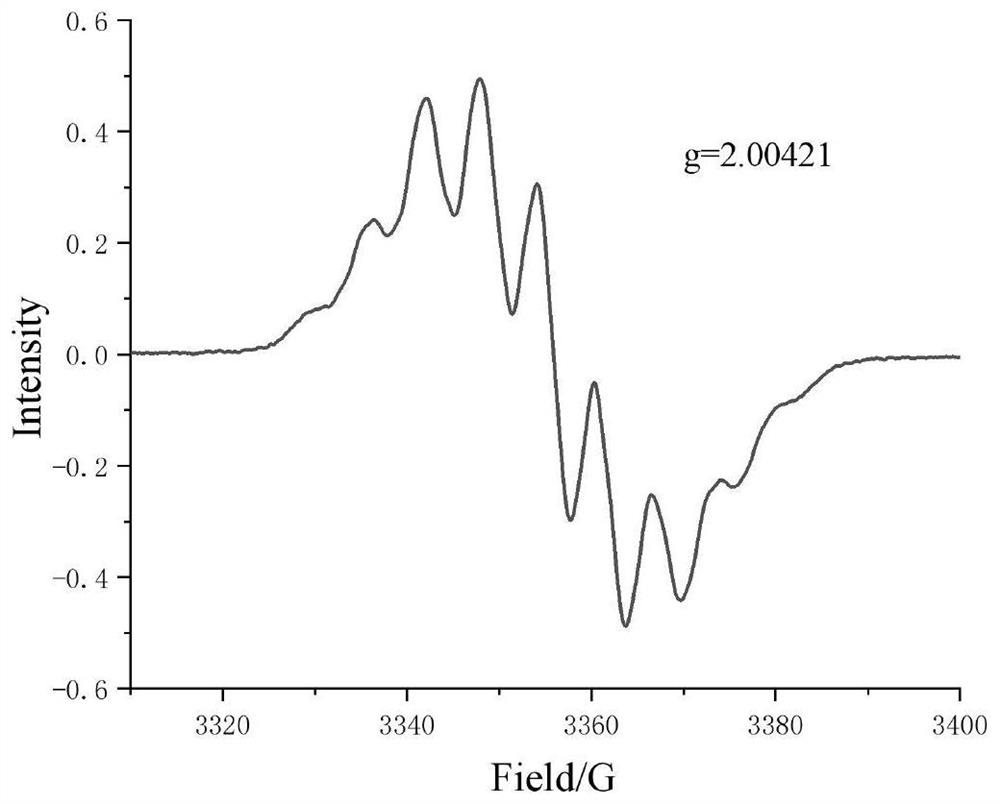
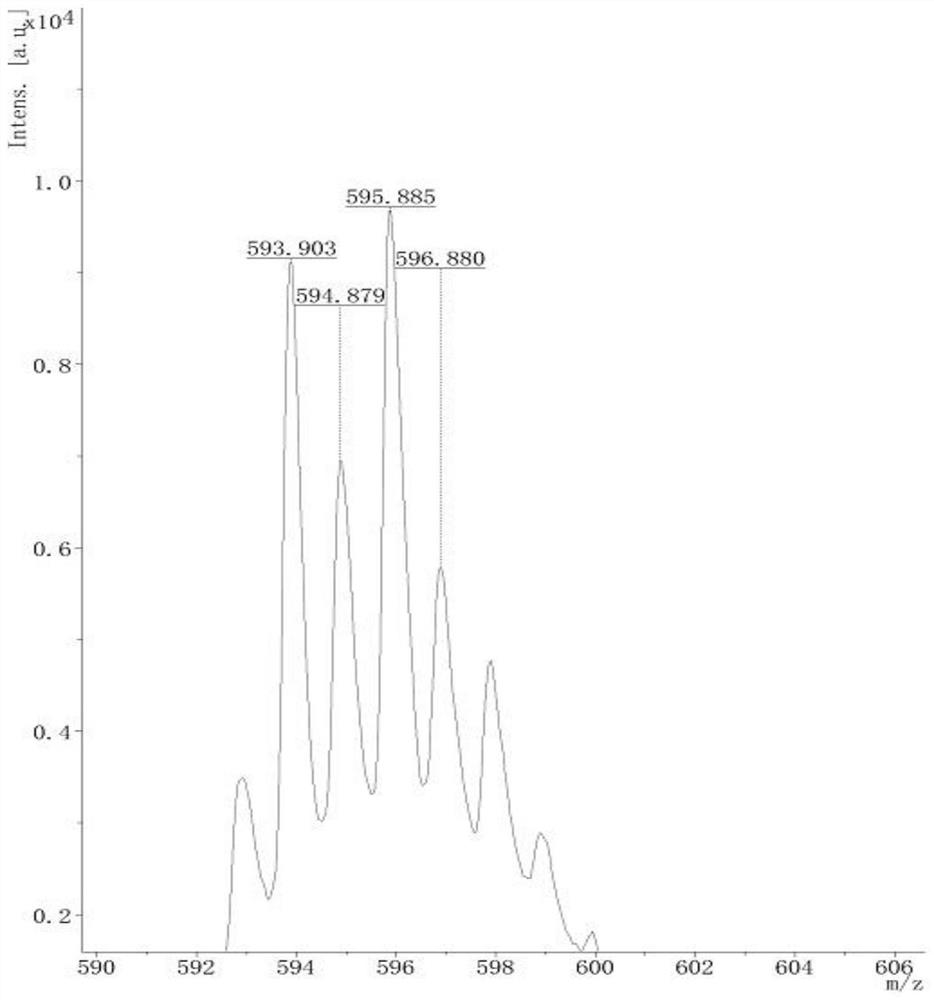
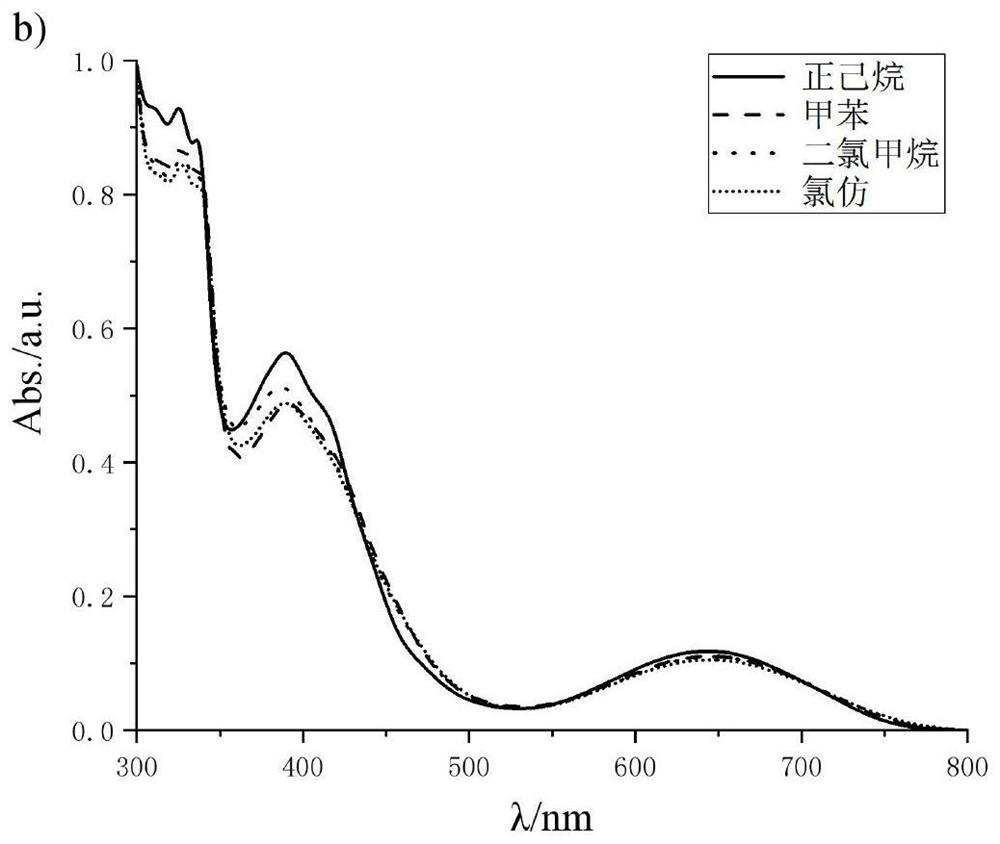
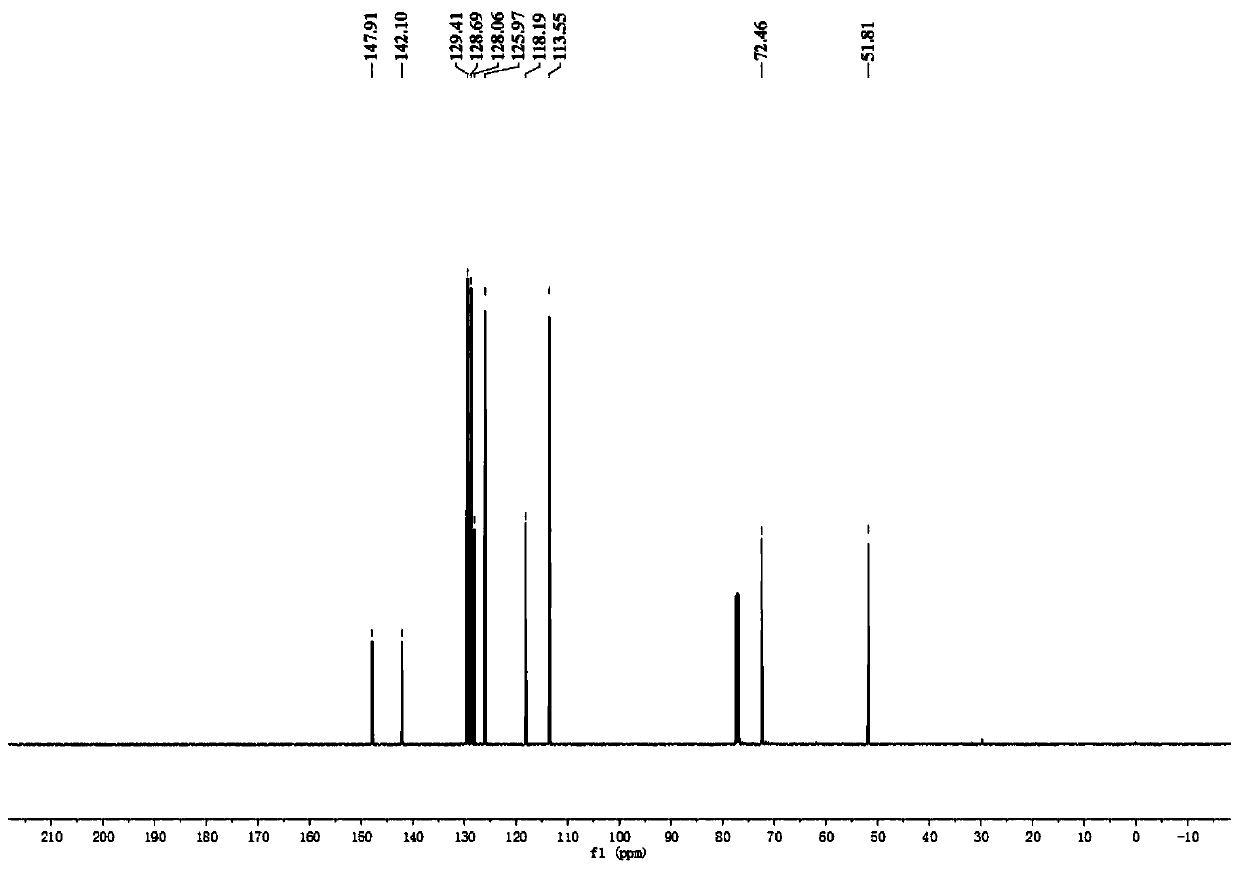
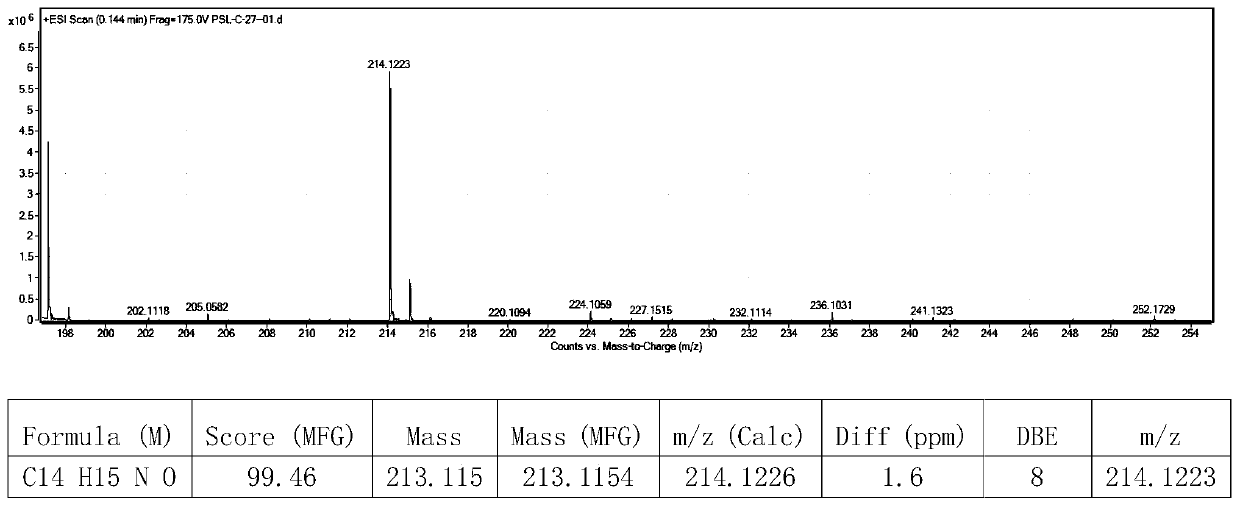
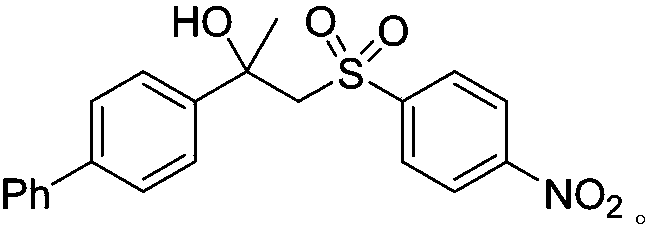
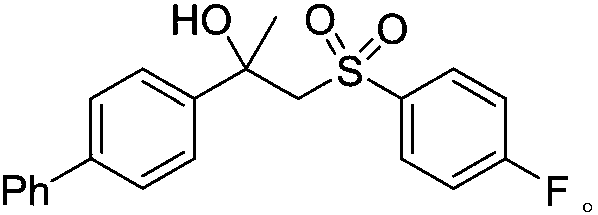
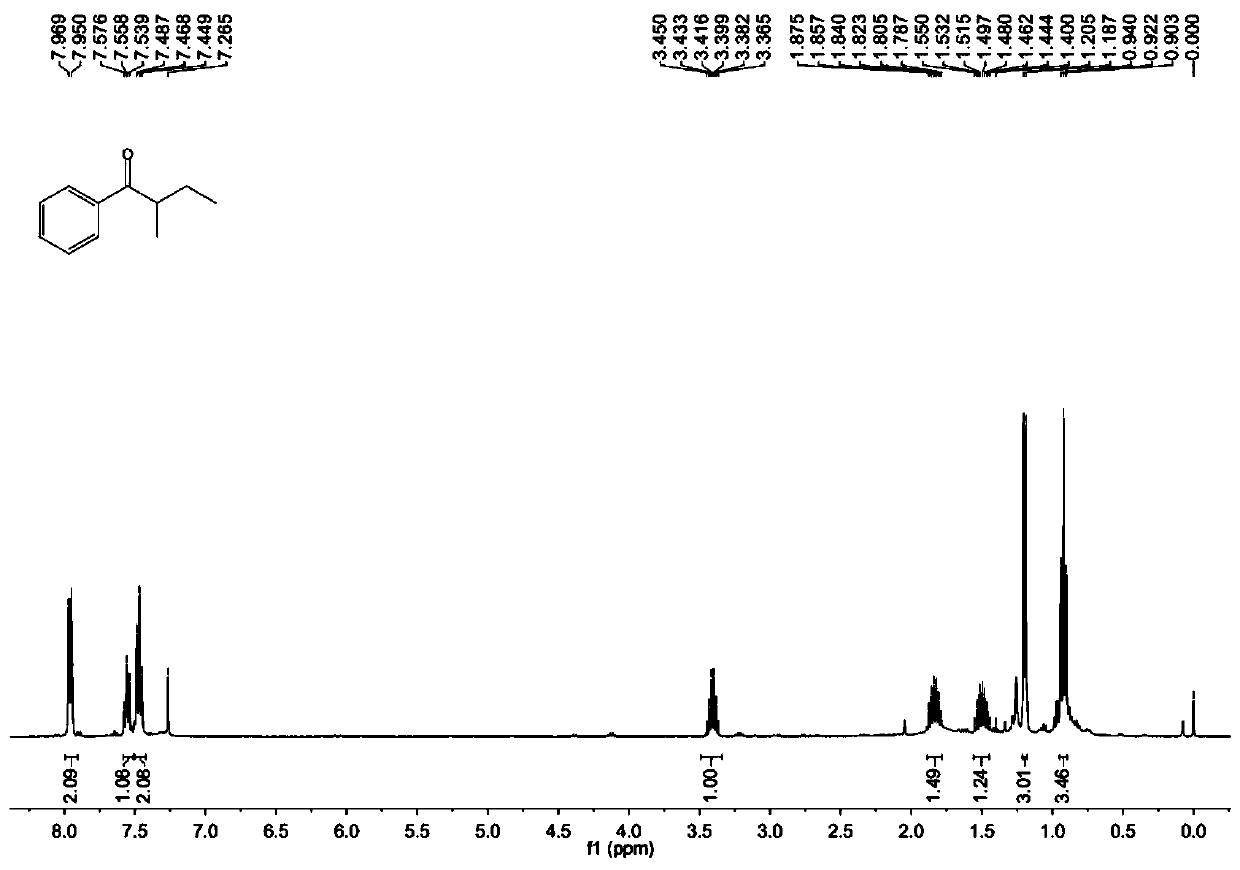
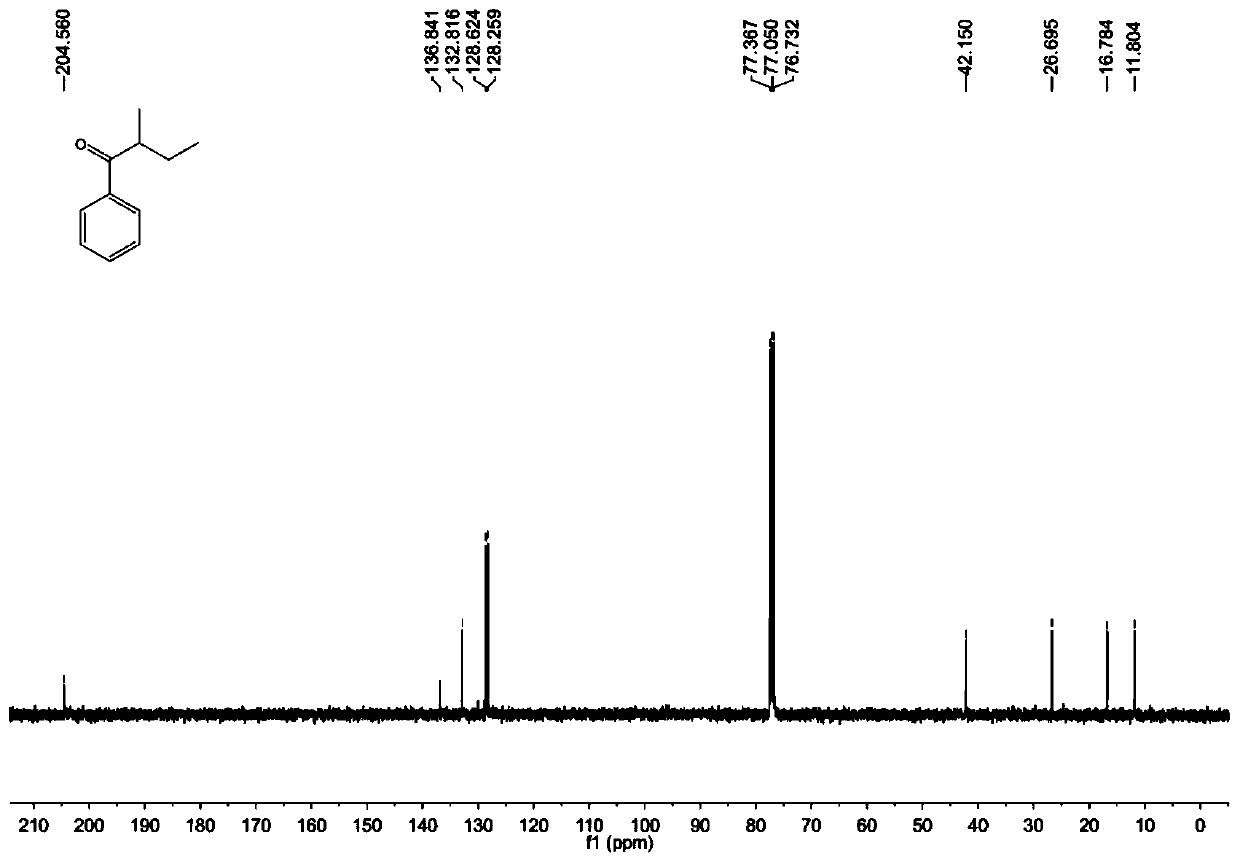
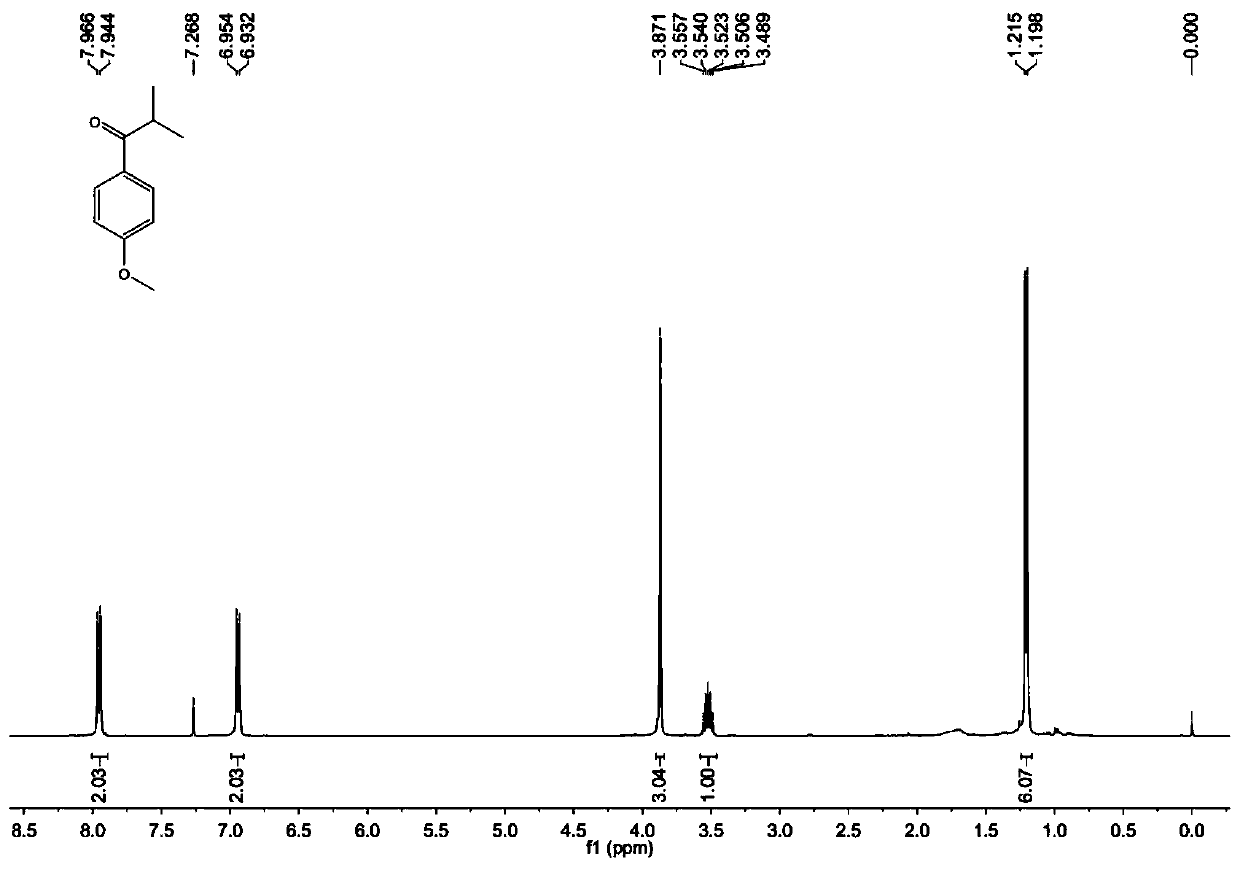
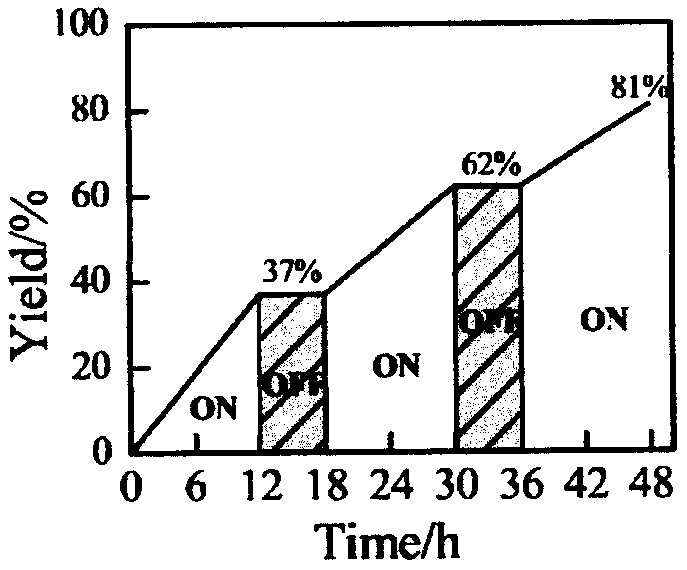
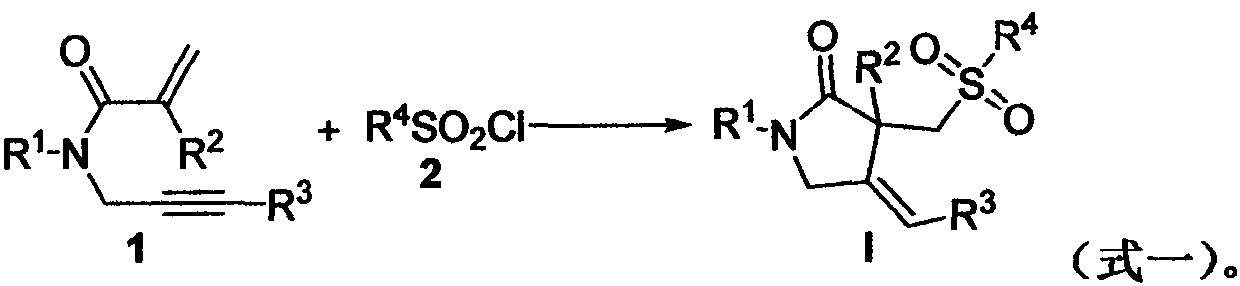
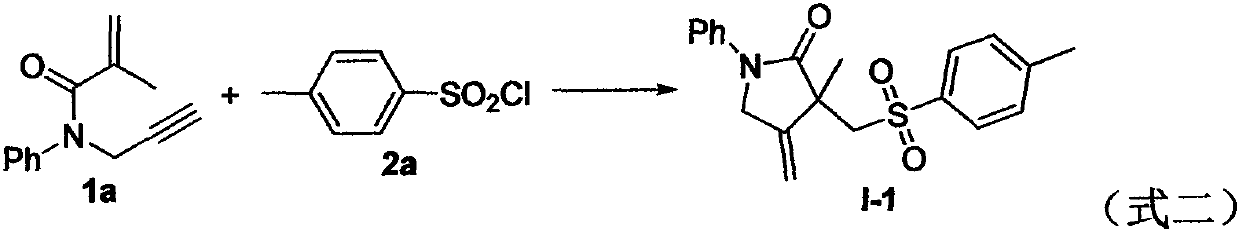
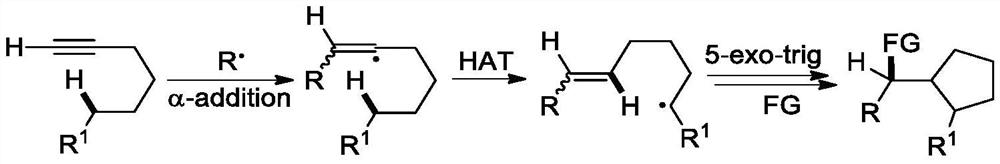
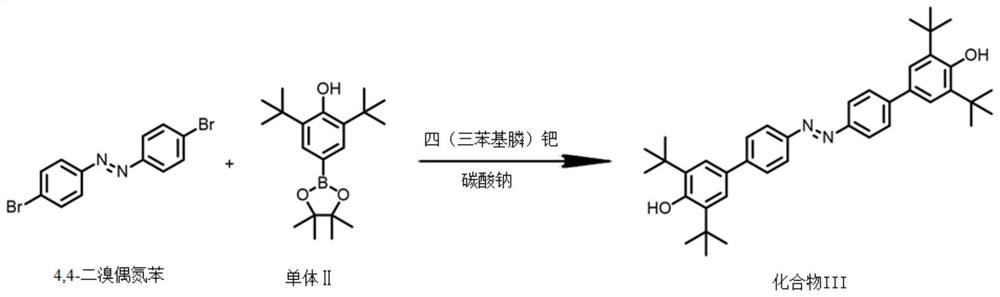
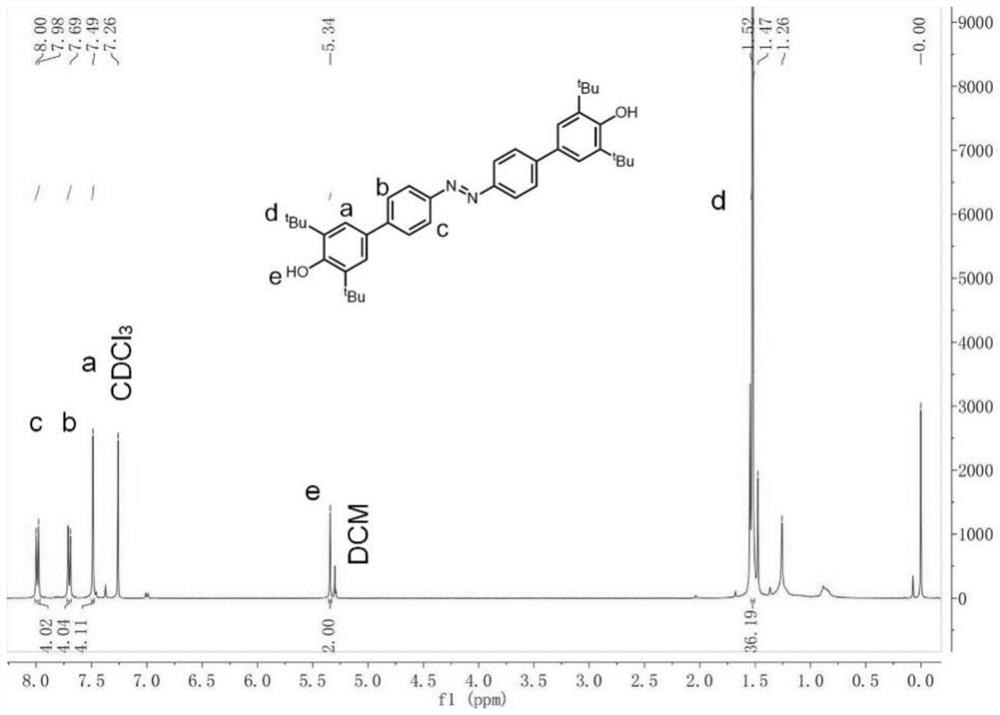
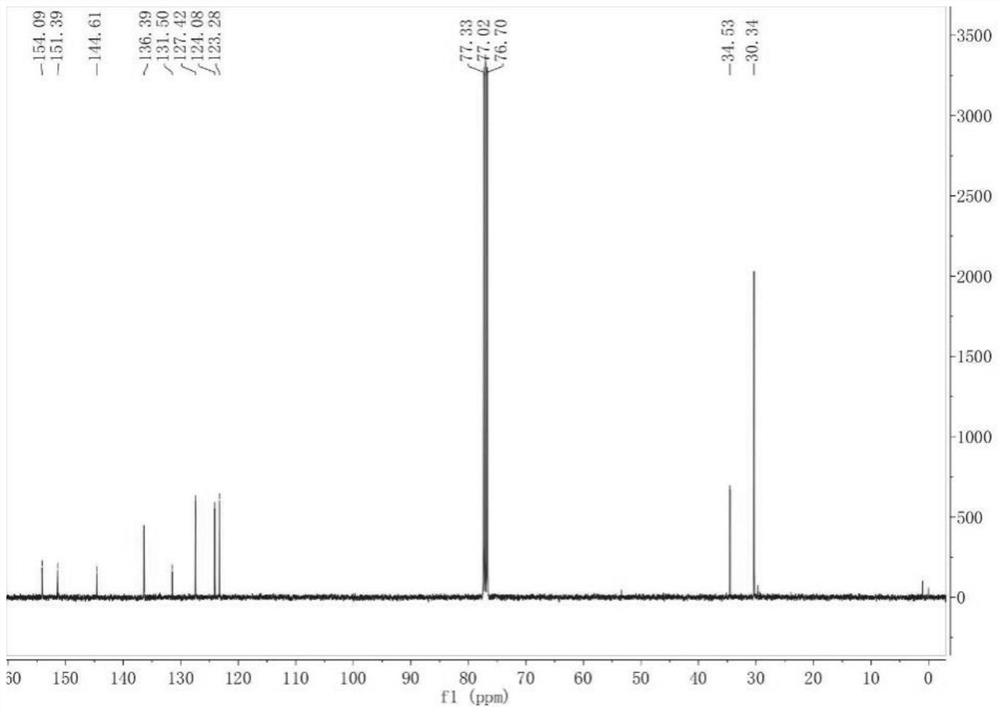
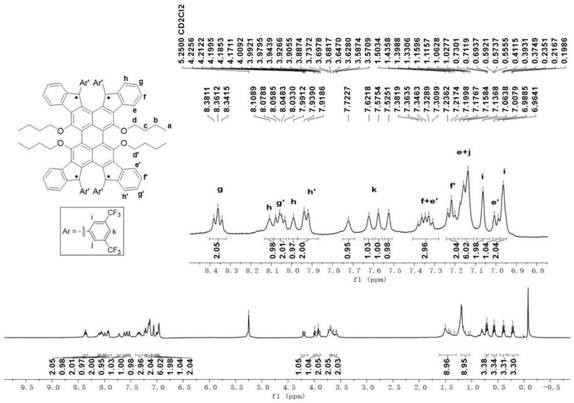
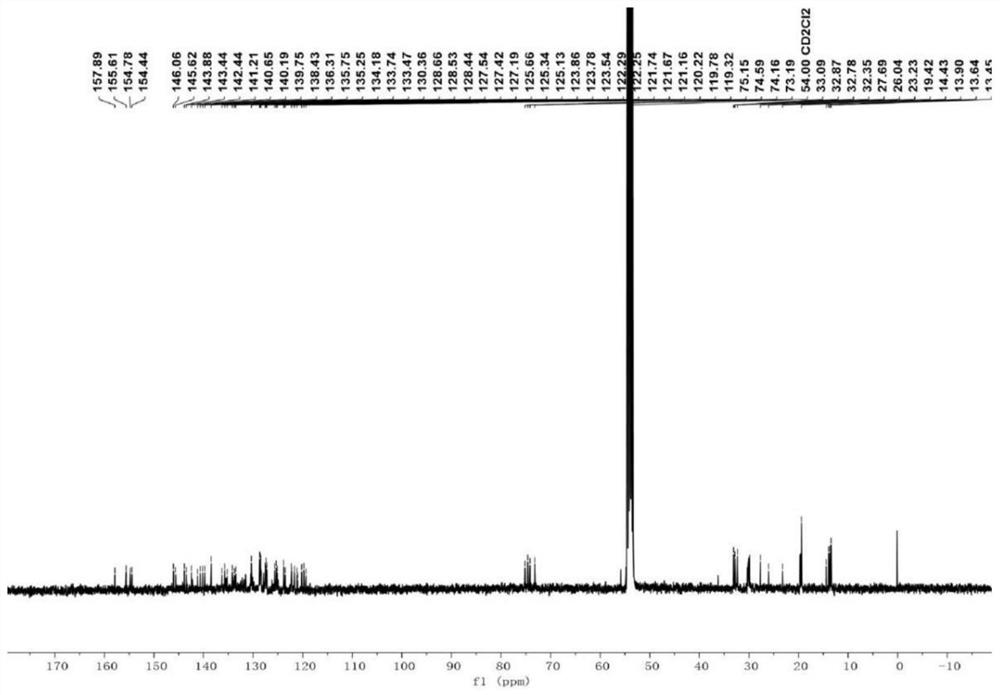
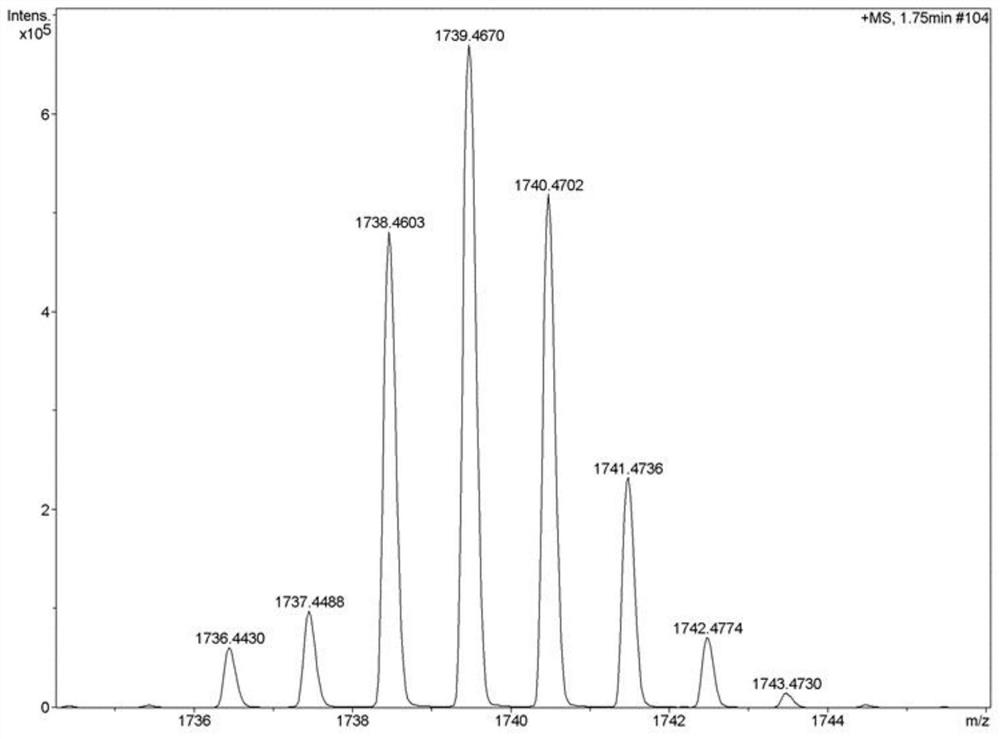
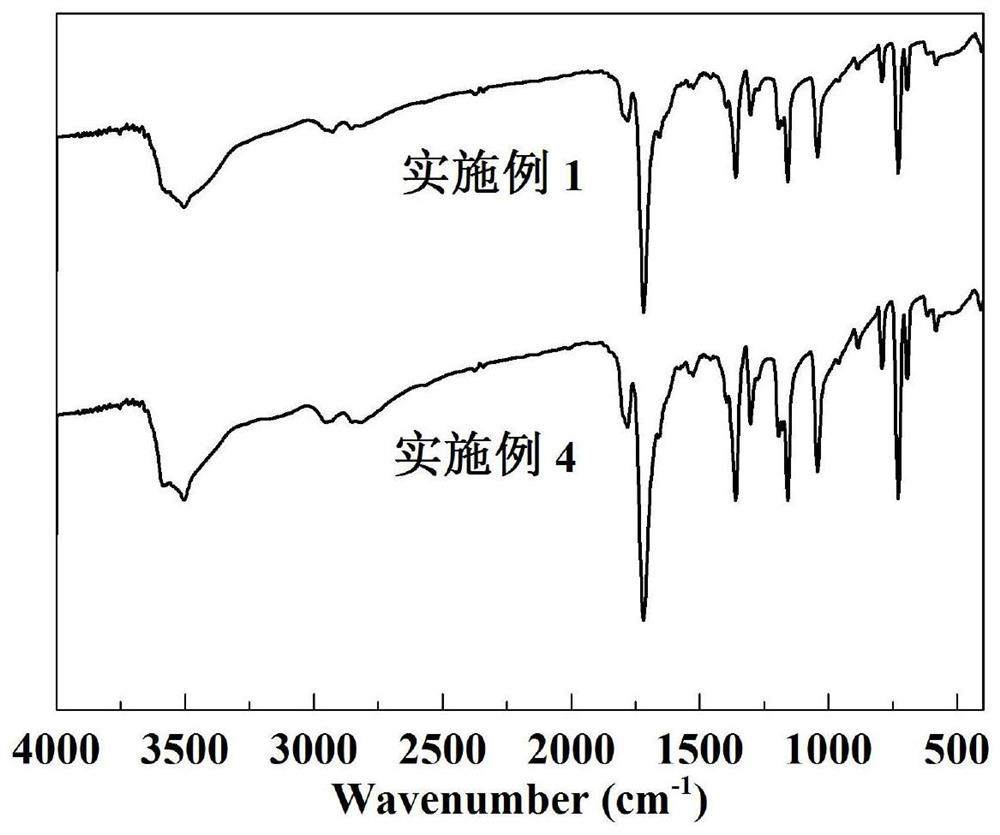
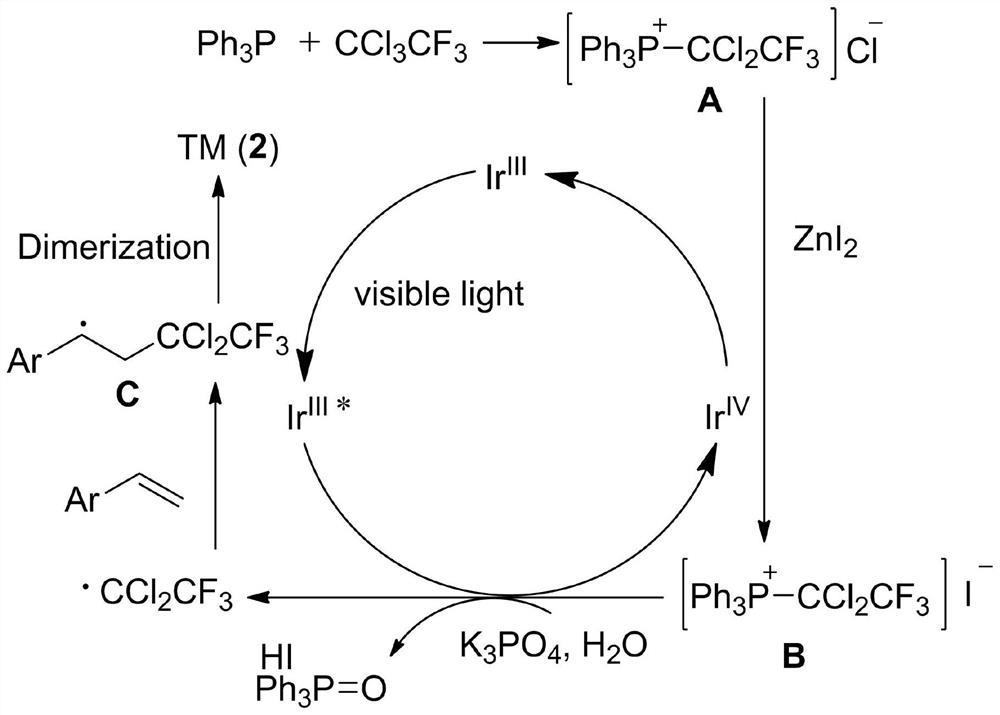
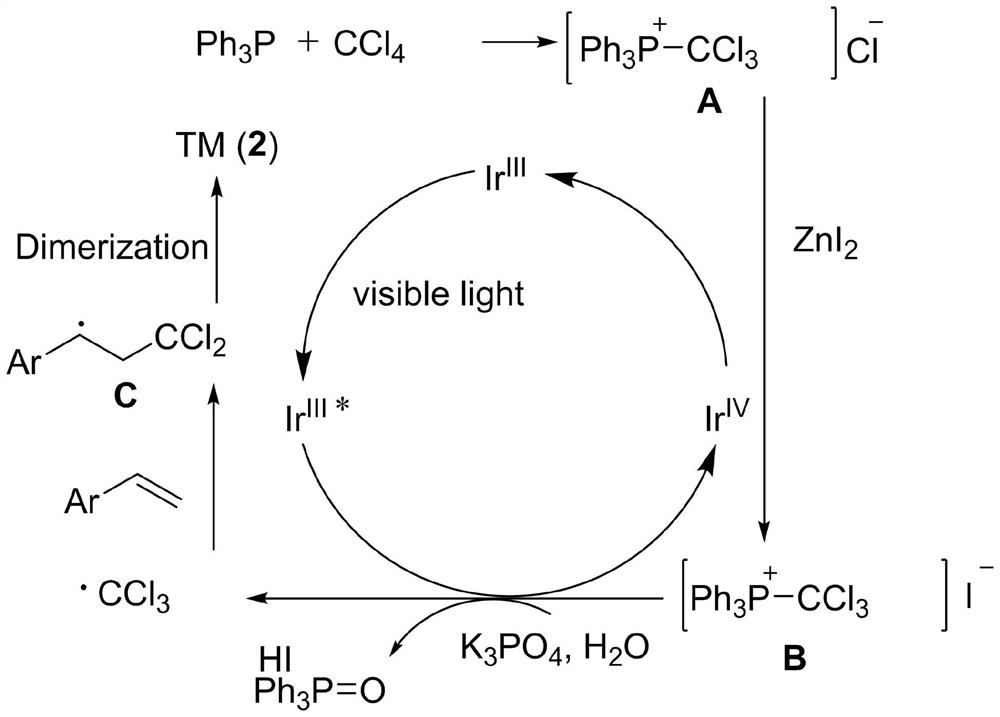
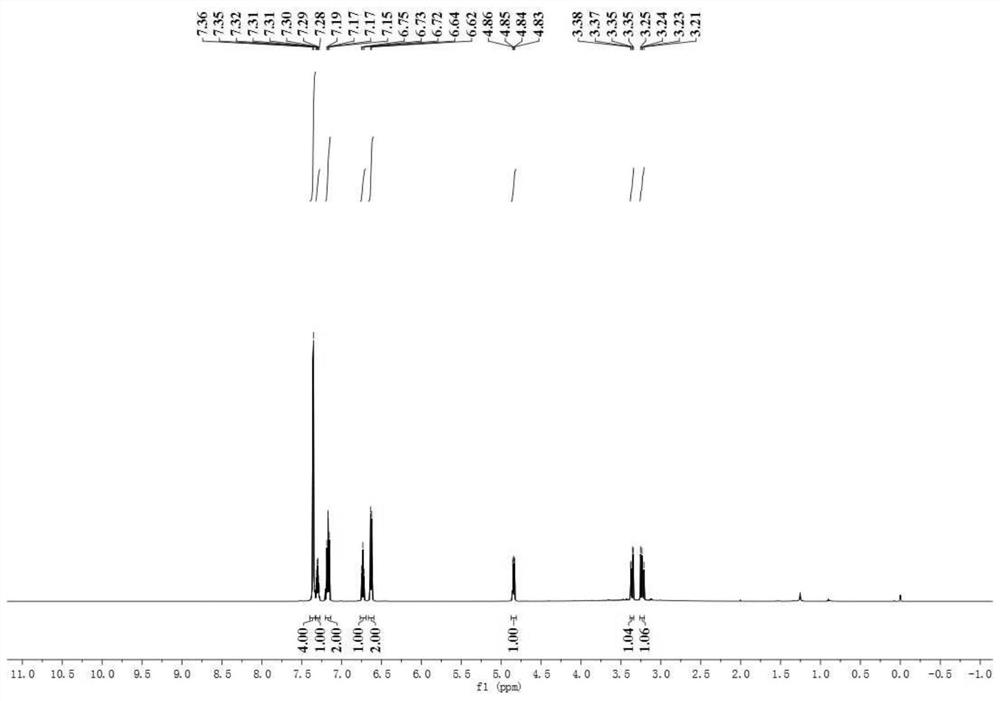
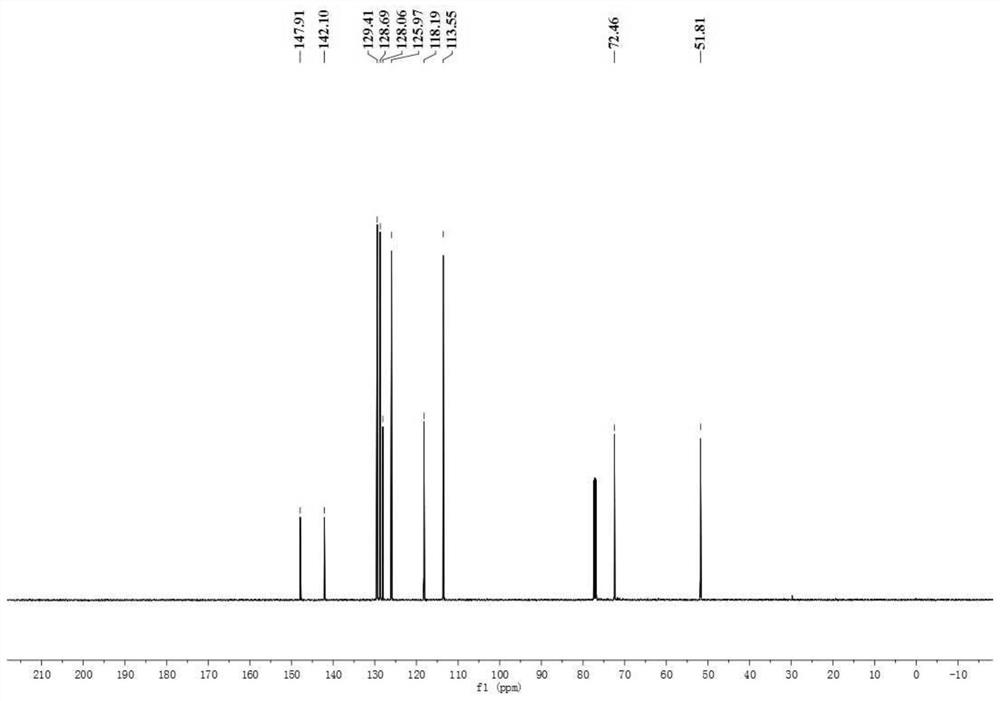
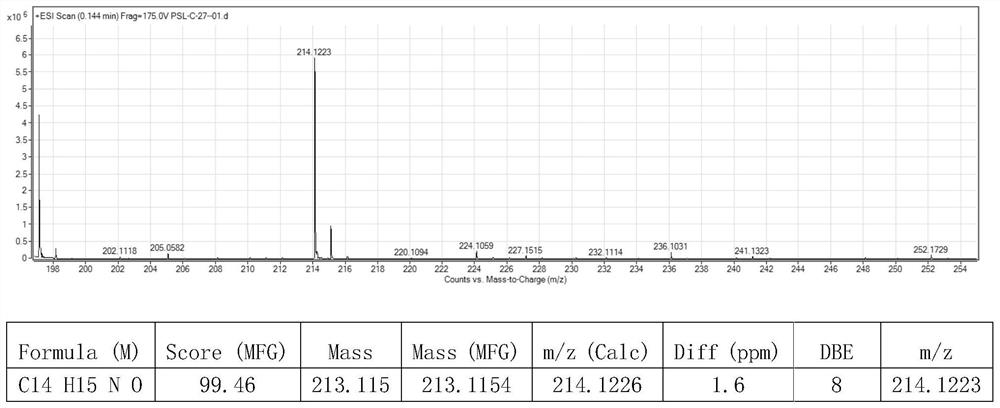
A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound
ActiveCN110878036BImprove compatibilityReduce usageOrganic compound preparationOrganic free radical generationHalohydrocarbonSilanes
Owner:TAIZHOU UNIV
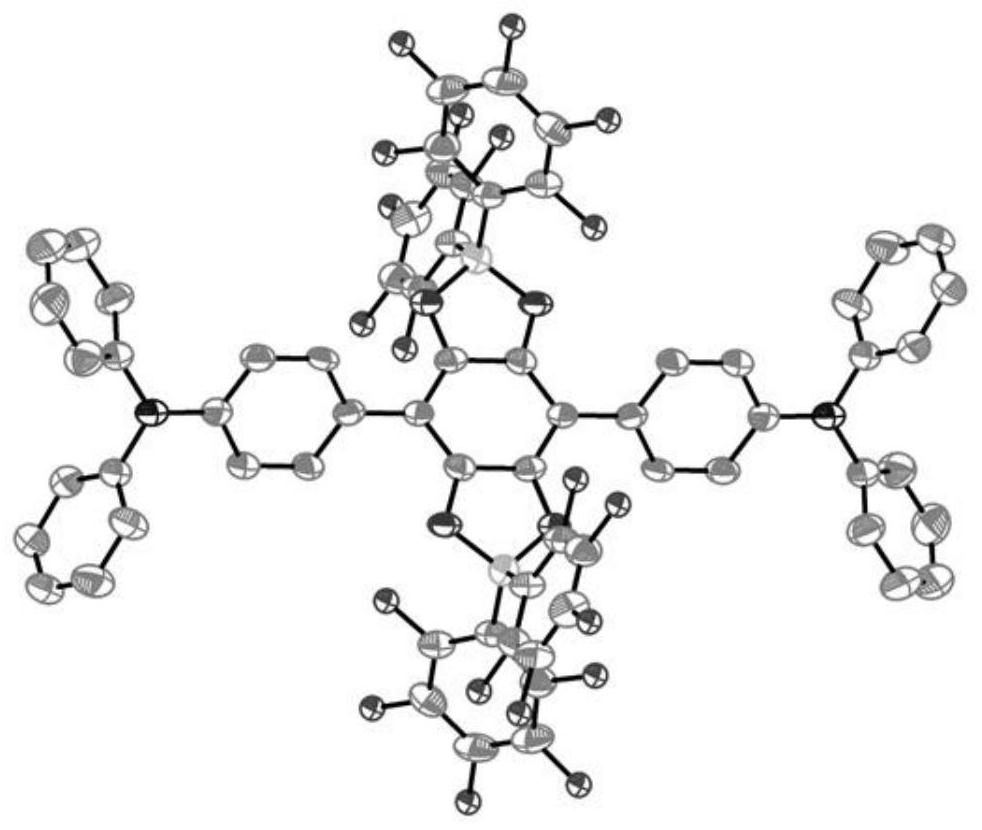
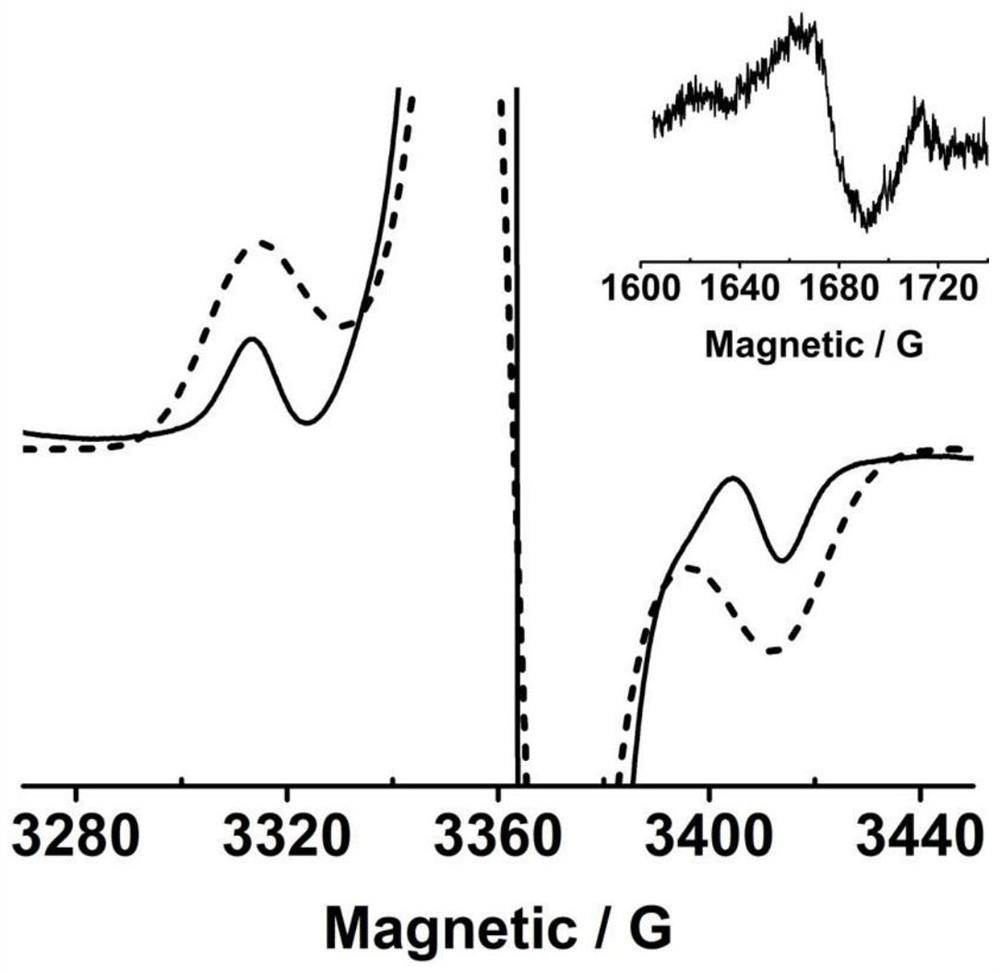
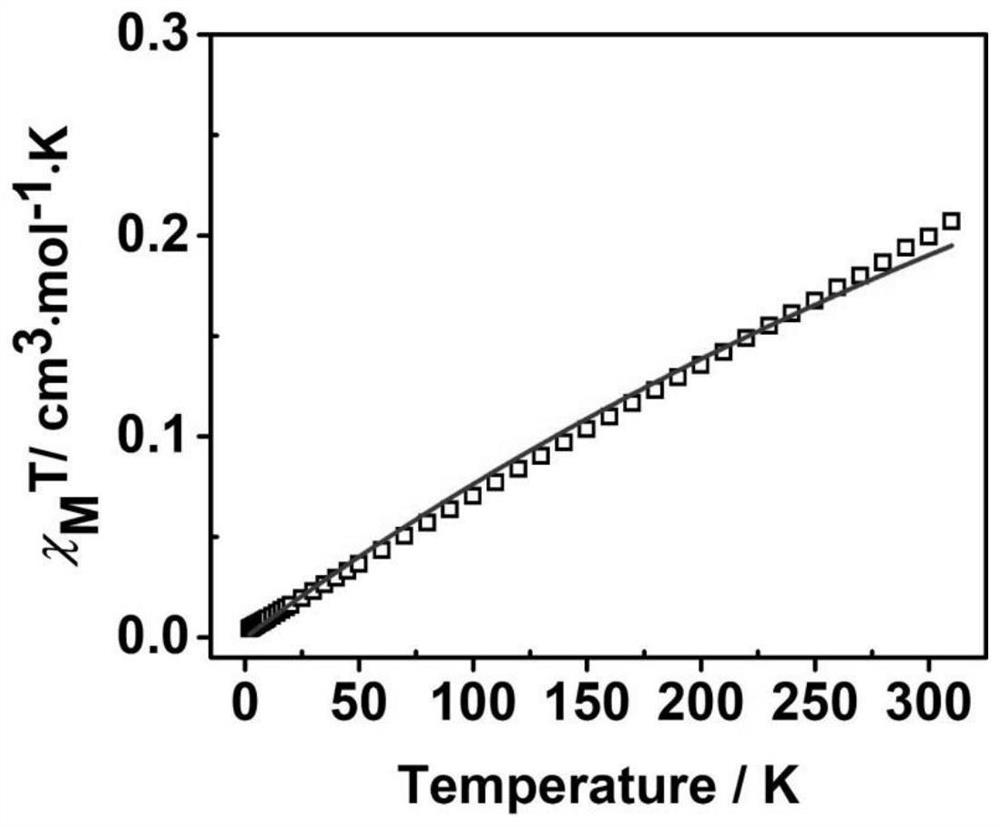
Neutral cross-conjugated biradical based on intramolecular ion pair and preparation method of neutral cross-conjugated biradical
PendingCN114804991AReduce usageThe synthetic route is simple and reliableOrganic free radical generationGroup 3/13 element organic compoundsOrganic solventPhysical chemistry
The invention discloses an intramolecular ion pair-based neutral cross-conjugated biradical and a preparation method thereof, and the preparation method comprises the following step: reacting a neutral precursor and Lewis acid in an organic solvent under the protection of inert gas. The synthesis method is simple and reliable, not only opens up a new way for constructing neutral double free radicals, but also provides a new opportunity for potential application of Lewis acid in the field of free radical chemistry.
Owner:NANJING UNIV
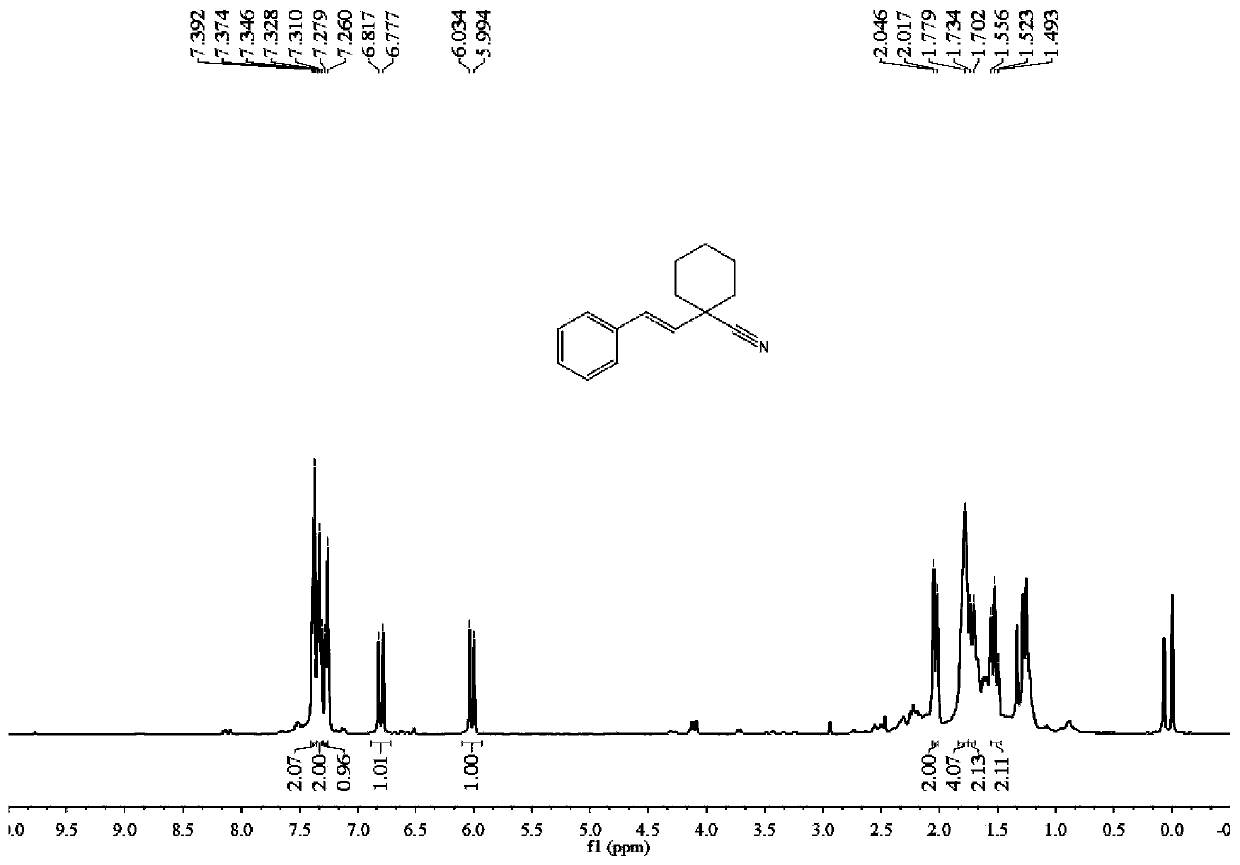
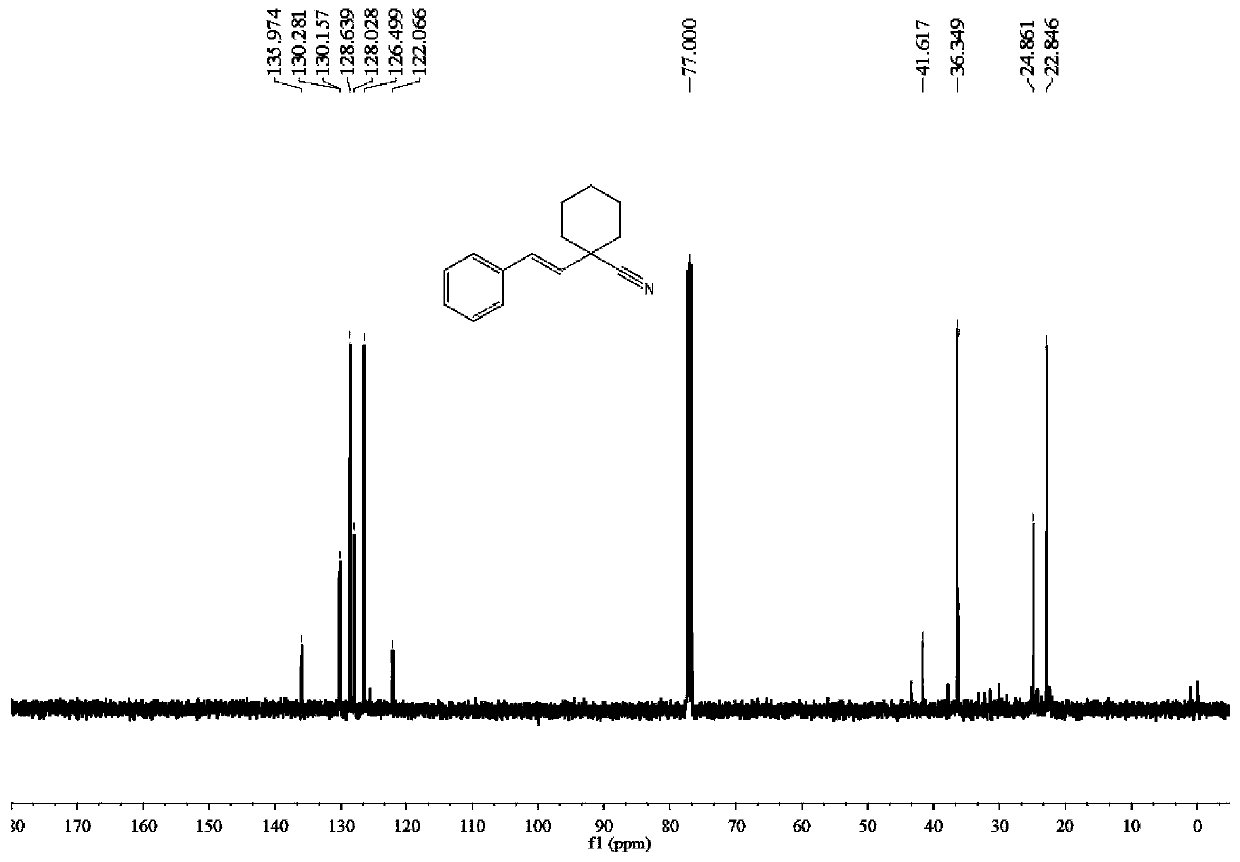
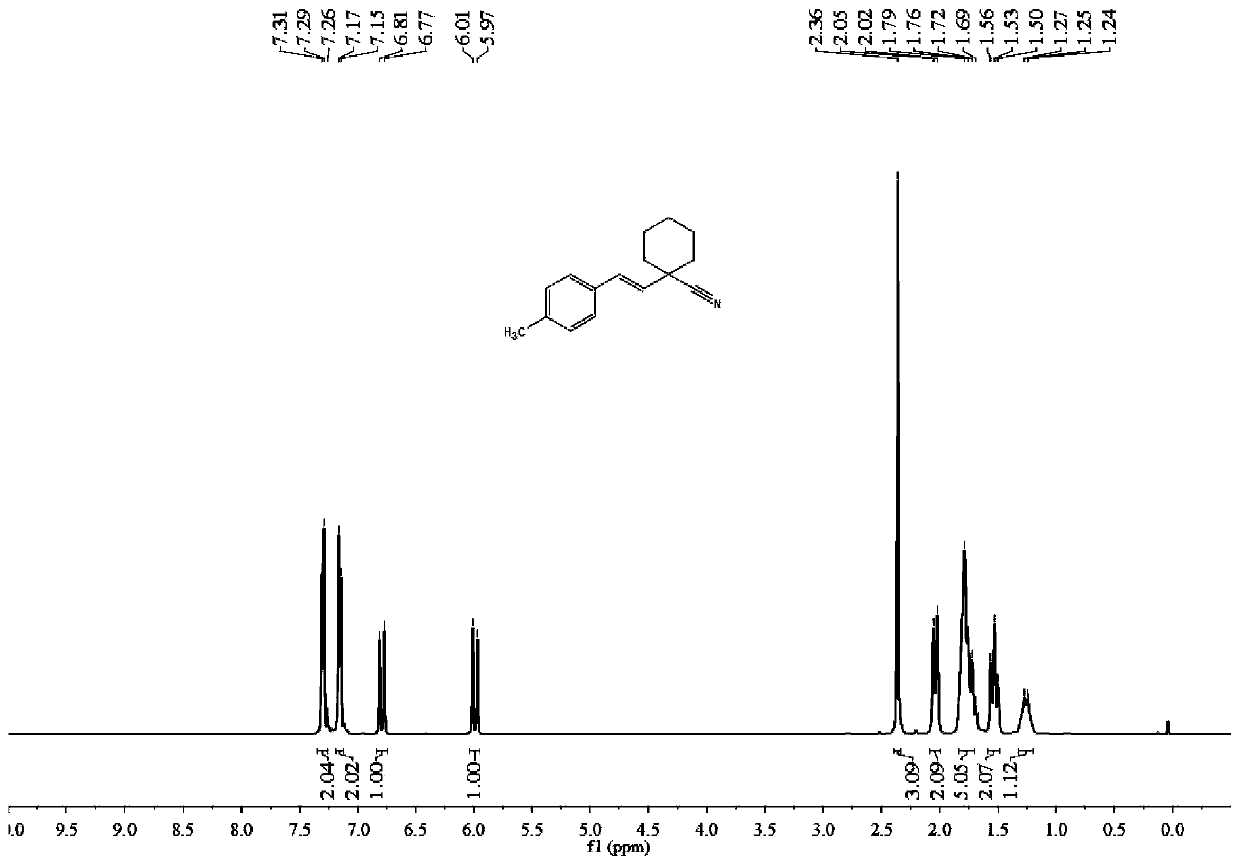
Synthesis method of (E)-1-styrylcyclohexane-1-nitrile compound
ActiveCN111499541ALow costAvoid it happening againCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystBiochemical engineering
The invention discloses a synthesis method of (E)-1-styrylcyclohexane-1-nitrile compounds, wherein the synthesis method comprises the step: carrying out one-pot reaction on styrene compounds and ACCNin a DMSO solvent system under the catalysis of elemental iodine to obtain the (E)-1-styrylcyclohexane-1-nitrile compound. The method does not need to adopt a metal catalyst and a toxic solvent, is beneficial to environmental protection, mild in reaction condition, simple in step, high in target product yield, high in atom utilization rate and beneficial to industrial production.
Owner:新疆普禾粟新型环保材料有限公司
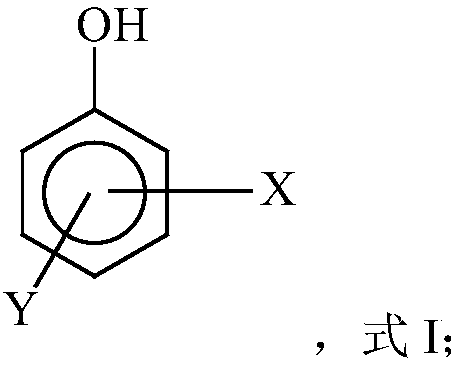
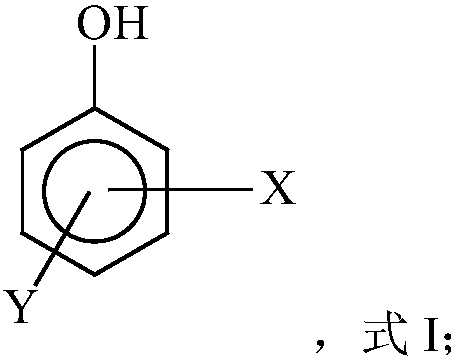
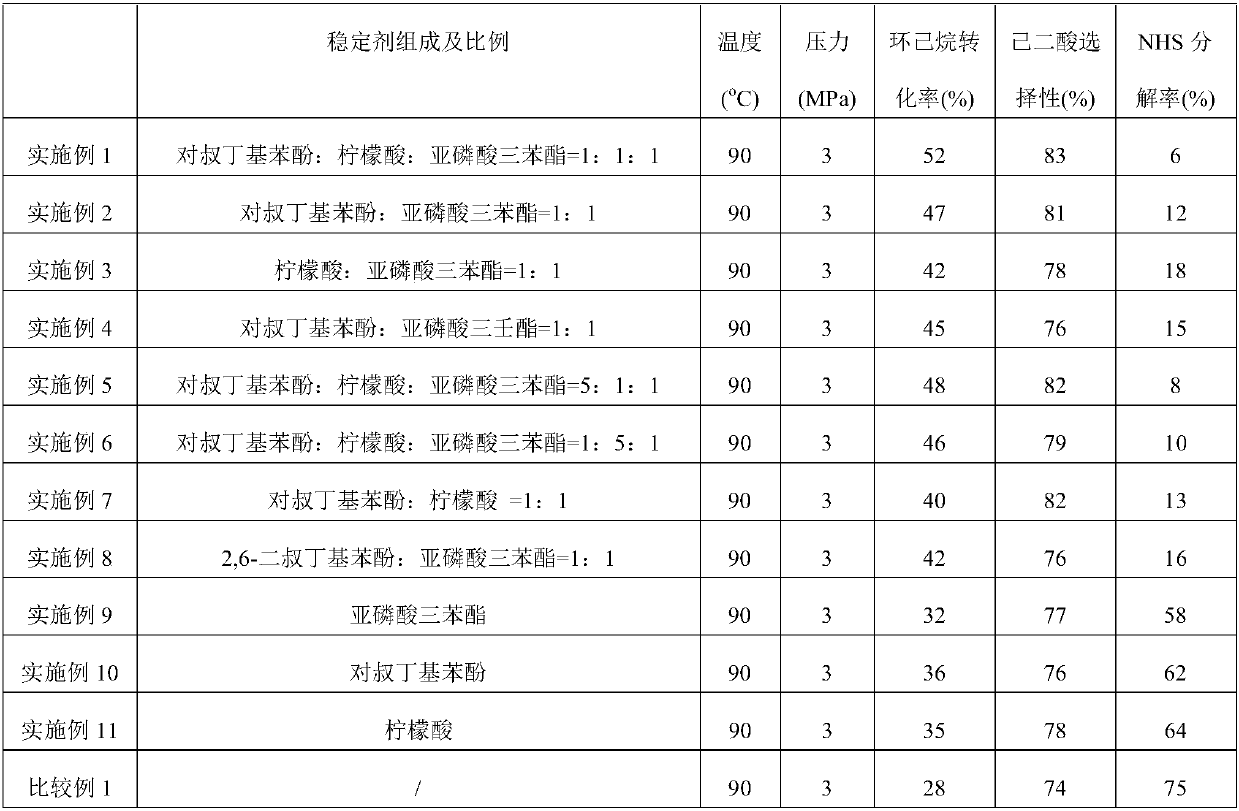
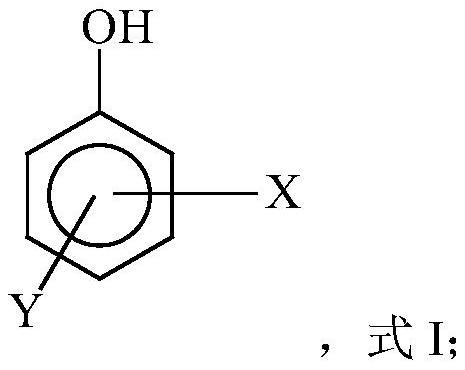
Free radical protective agent for preparing hexanedioic acid through direct oxidation of cyclohexane
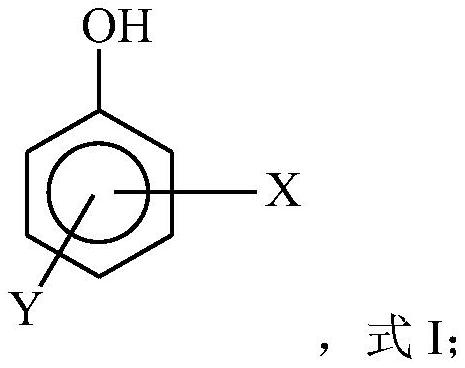
ActiveCN109096096AReduce inactivation rateHigh inactivation rateOrganic compound preparationOrganic free radical generationPhenolCitric acid
The invention relates to a free radical protective agent for preparing hexanedioic acid through direct oxidation of cyclohexane, and mainly solves the problem in the prior art that a free radical catalyst inactivates and decomposes easily in the reaction of preparing the hexanedioic acid through the direct oxidation of the cyclohexane. The free radical protective agent comprises citric acid and / orat least one of substituted phenol shown in the formula in the description, wherein the technical scheme that X and Y are independently selected from H or tertiary butyl, and X and Y are not used inH at the same time better solves the problem, and the free radical protective agent for preparing the hexanedioic acid through the direct oxidation of the cyclohexane can be applied to the industrialproduction of preparing the hexanedioic acid through the direct oxidation of the cyclohexane.
Owner:CHINA PETROLEUM & CHEM CORP +1
Free Radical Protection Agent for Direct Oxidation of Cyclohexane to Adipic Acid
ActiveCN109096096BReduce inactivation rateHigh inactivation rateOrganic compound preparationOrganic free radical generationCombinatorial chemistryAdipic acid
The invention relates to a free radical protecting agent for preparing adipic acid through the direct oxidation of cyclohexane, which mainly solves the problem in the prior art that the free radical catalyst is easily deactivated and decomposed in the reaction of preparing adipic acid through the direct oxidation of cyclohexane. By adopting cyclohexane direct oxidation system adipic free radical protection agent, including at least one of citric acid and / or substituted phenol shown in the following formula; wherein, X and Y are independently selected from H or tert-butyl, And the technical scheme that X and Y are not H at the same time solves this problem well, and can be used in the industrial production of adipic acid by direct oxidation of cyclohexane.
Owner:CHINA PETROLEUM & CHEM CORP +1
Organic light-emitting nitrogen free radical molecule based on nitrogen heterocyclic structure and preparation method of organic light-emitting nitrogen free radical molecule
ActiveCN114804992AEasy to operateEasy to industrializeOrganic free radical generationLuminescent compositionsSingle electronElectronegativity
The invention belongs to the technical field of organic light-emitting materials, and provides an organic light-emitting nitrogen free radical molecule based on a nitrogen heterocyclic structure and a preparation method of the organic light-emitting nitrogen free radical molecule. The organic light-emitting nitrogen free radical molecule based on the nitrogen heterocyclic structure has a structure as shown in a formula I which is described in the specification. The center position of the organic light-emitting nitrogen free radical molecule is a six-membered nitrogen heterocyclic free radical, the six-membered nitrogen heterocyclic free radical has four nitrogen atoms, the six-membered nitrogen heterocyclic free radical is delocalized on two nitrogen atoms through resonance, and the other two nitrogen atoms and two carbon atoms can be connected with substituent groups respectively, so that the organic light-emitting nitrogen free radical molecule can be formed. The modification on organic light-emitting nitrogen free radical molecules is realized. Single electrons are delocalized on six-membered heterocycles, so that the density of the single electrons is effectively dispersed; meanwhile, as many as four nitrogen atoms exist in a six-membered heterocyclic ring, and the electronegativity of the nitrogen atoms is greater than that of carbon atoms, so that the action of single electrons and atomic nuclei is tighter than that of carbon free radicals, and the single electrons and the atomic nuclei are not easy to react with other substances, so that the stability is relatively good under an illumination condition.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
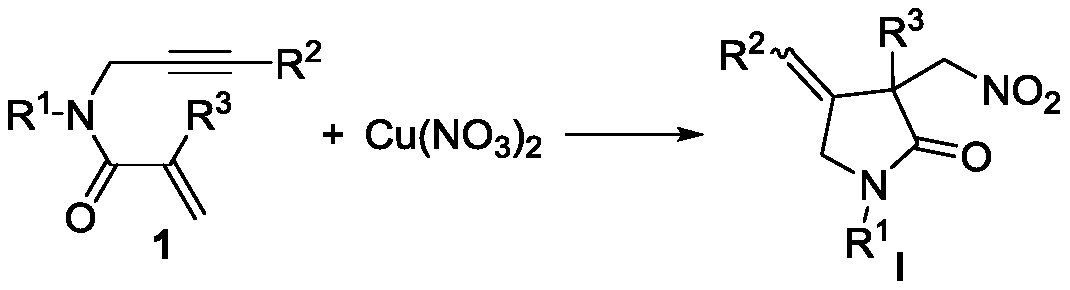
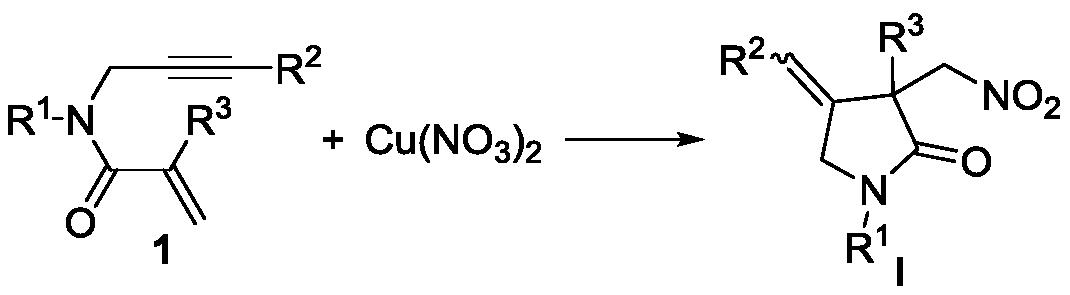
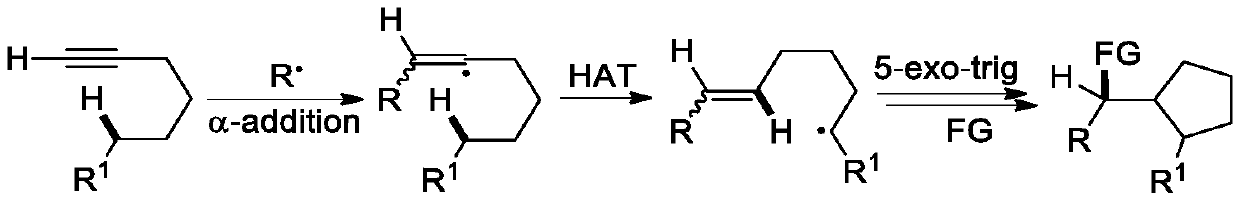
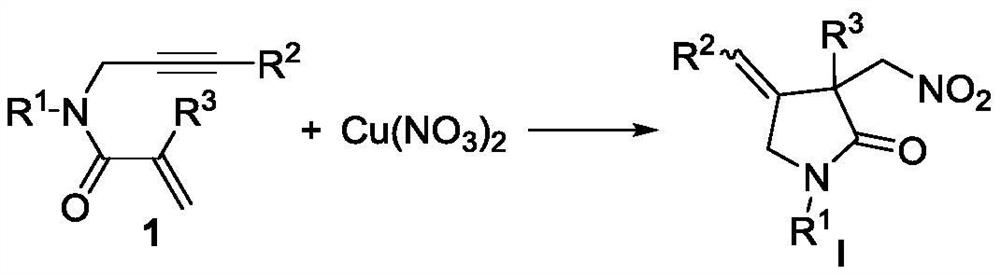



![A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound](https://images-eureka.patsnap.com/patent_img/81439e7e-9443-4db4-a93b-2160ea0e9503/BDA0002197068410000021.png)
![A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound](https://images-eureka.patsnap.com/patent_img/81439e7e-9443-4db4-a93b-2160ea0e9503/BDA0002197068410000031.png)
![A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound A kind of preparation method of [(1,2-disulfonyl) ethyl] arene compound](https://images-eureka.patsnap.com/patent_img/81439e7e-9443-4db4-a93b-2160ea0e9503/BDA0002197068410000041.png)