Neutral cross-conjugated biradical based on intramolecular ion pair and preparation method of neutral cross-conjugated biradical
A cross-conjugation and free-radical technology, applied in the field of neutral cross-conjugated double-radical and its preparation, can solve the problems of hindering the research and application of double-radical molecules, difficult separation and crystallization, complicated reaction process, etc. The effect of simple and reliable route, simple preparation method and broad application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a glove box, the neutral precursor (R1~R4 are all H) (0.2mmol, 136.6mg), tris(pentafluorophenyl)borane (209.9mg, 0.41mmol) and 25mL of anhydrous and oxygen-free toluene were placed In a 100 mL Schlenk bottle, the mixture was heated to 135° C. under a nitrogen atmosphere and stirred for 12 h under reflux. After the reaction is over, filter through an organic filter to obtain a yellow-brown solution and concentrate it to about 15 mL, add an appropriate amount of n-hexane, and then place it at -40°C for low-temperature crystallization, and finally obtain a solution suitable for single crystal X-ray diffraction. Dark yellow crystals. Yield: 91.75 mg, 34.9%.
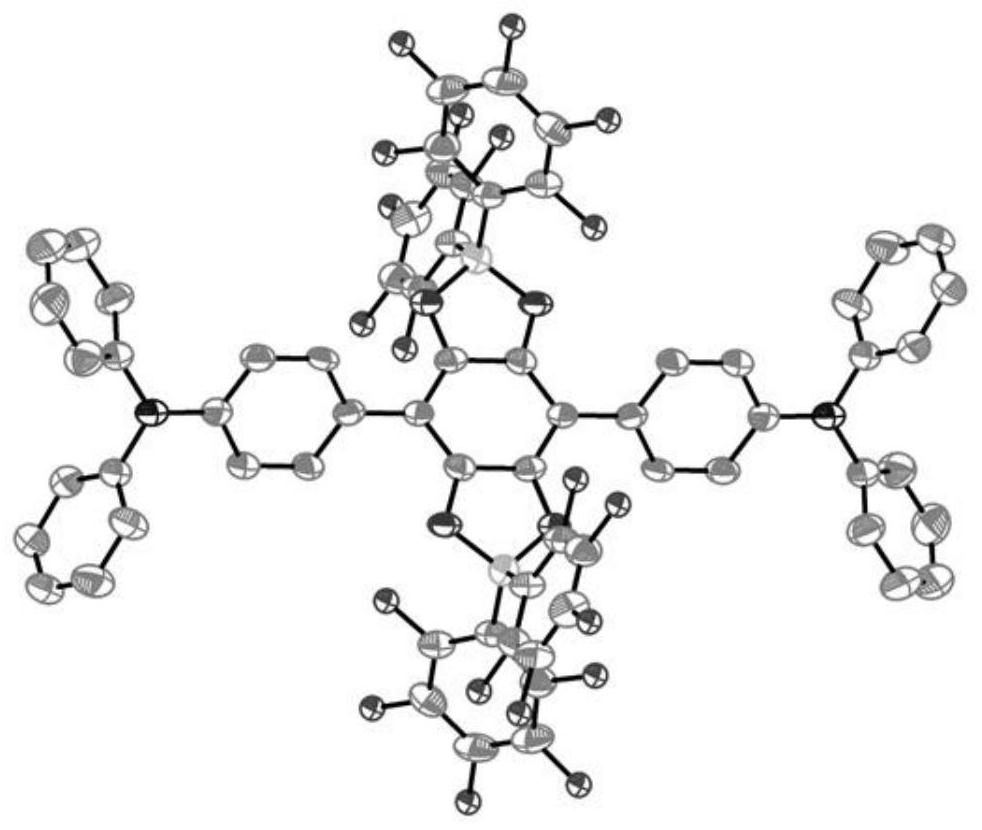
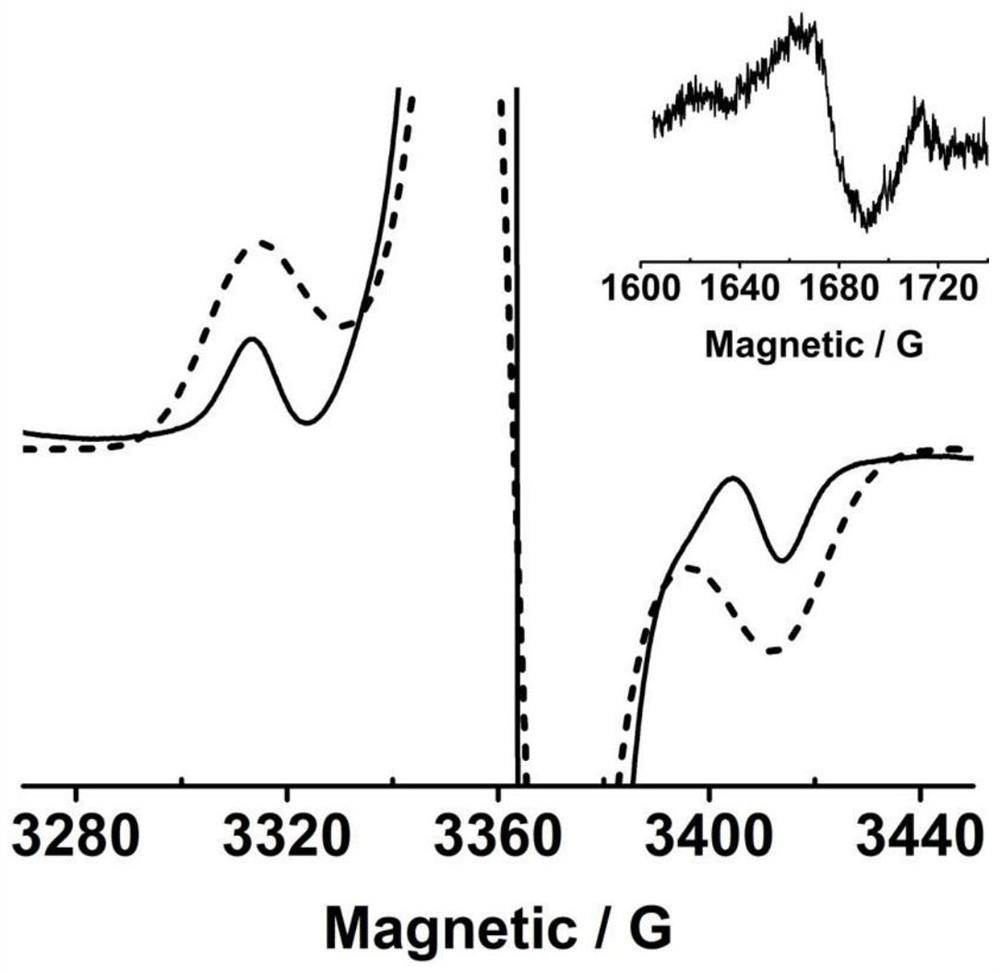
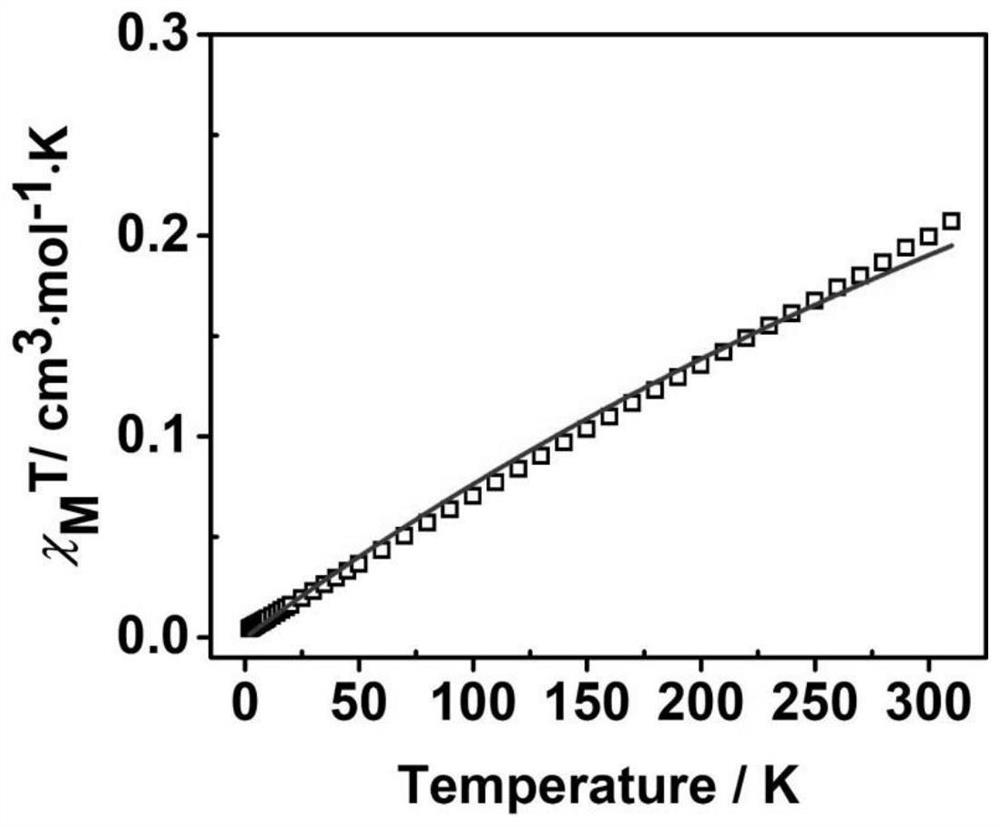
[0046] The result is as Figure 1-3 shown, figure 1 is the crystal structure of the synthesized neutral bis(triarylamine) diradical, which shows the formation of two planar five-membered B-heterocycles bridged by a benzene ring containing a donor group-bis(triarylamine) unit . C in the crystal 6 O 4 B 2 The ...
Embodiment 2
[0054] In the glove box, the neutral precursors (R1~R4 are all CH 3 ) (0.2 mmol, 147.8 mg), tris(pentafluorophenyl) borane (209.9 mg, 0.41 mmol) and 25 mL of anhydrous and oxygen-free toluene were placed in a 100 mL Schlenk flask, heated to 140 ° C under nitrogen atmosphere and stirred for reaction 48h. After the reaction is over, filter through an organic filter to obtain an orange-yellow solution and concentrate it to about 15 mL, add an appropriate amount of n-hexane, and then place it at -40°C for low-temperature crystallization, and finally obtain a solution suitable for single crystal X-ray diffraction. Orange-yellow crystals. Yield: 96.22 mg, 35.1%.
[0055] The result is as Figure 4-6 shown, Figure 4 is the crystal structure of the synthesized neutral bis(triarylamine) diradical, it can be seen that two planar five-membered B- Heterocycle. C in the crystal 6 O 4 B 2 The dihedral angle between the plane of the unit and the plane of the adjacent benzene ring i...
Embodiment 3
[0063] In a glove box, the neutral precursor (R1~R4 are all tBu) (0.2mmol, 170.2mg), tris(pentafluorophenyl)borane (209.9mg, 0.41mmol) and 25mL of anhydrous and oxygen-free toluene were placed In a 100 mL Schlenk bottle, the reaction was heated to 150° C. under a nitrogen atmosphere and stirred for 36 h. After the reaction is over, filter through an organic filter to obtain a dark yellow solution and concentrate it to about 15 mL, add an appropriate amount of n-hexane, and then place it at -40°C for low-temperature crystallization, and finally obtain a solution suitable for single crystal X-ray diffraction. Dark yellow crystals. Yield: 88.95 mg, 28.9%.
[0064] The result is as Figure 7-8 shown, Figure 7 is the crystal structure of the synthesized neutral bis(triarylamine) diradical, it can be seen that two planar five-membered B- Heterocycle. C in the crystal 6 O 4 B 2 The dihedral angle between the plane of the unit and the plane of the adjacent benzene ring is app...
PUM
| Property | Measurement | Unit |
|---|---|---|
| magnetic susceptibility | aaaaa | aaaaa |
| magnetic susceptibility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com