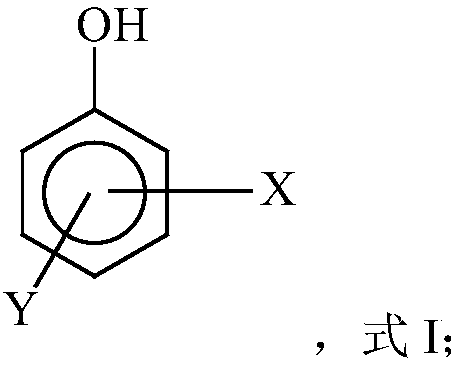
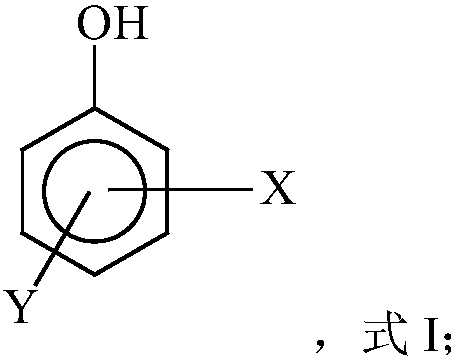
Free radical protective agent for preparing hexanedioic acid through direct oxidation of cyclohexane
A technology of free radicals and cyclohexane, applied in the field of free radical protection agents, can solve the problems of reducing the decomposition rate of free radical catalysts, easy deactivation and decomposition of free radical catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
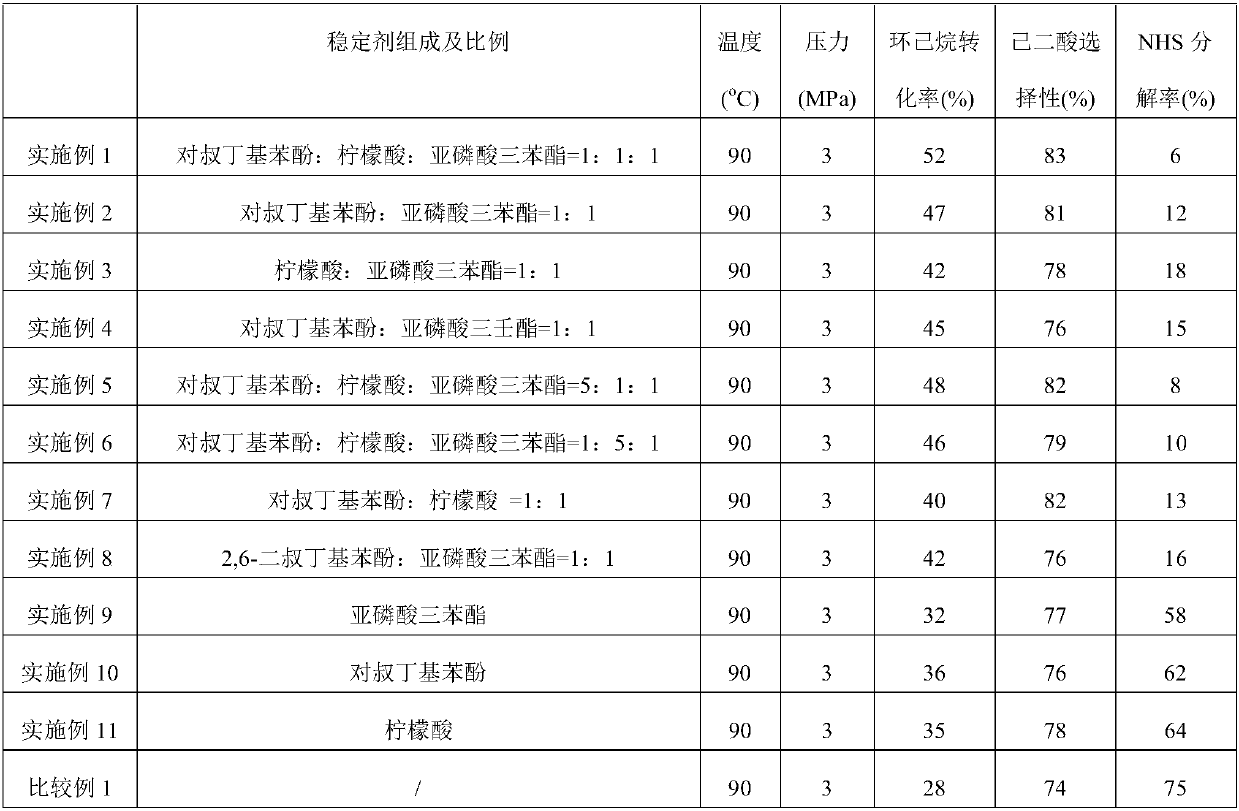
[0029] Add 5mol acetic acid, 0.02mol NHS, 0.01mol cobalt acetate, 0.01mol manganese acetate, 0.01mol copper acetate, 1mol cyclohexane, 0.01mol p-tert-butylphenol, 0.01mol citric acid and 0.01mol triphenyl phosphite to In a 1-liter high-pressure reactor (with a reflux condensing device, the reflux condensing device communicates with the atmosphere through a backup pressure valve), seal and stir, heat to 90°C, and continuously feed air at 5 liters / minute to control the pressure inside the kettle. 3MPa, after reacting for 5 hours, cool to room temperature, take out the reaction mixture for analysis, the analysis results: the conversion rate of cyclohexane is 52%, the selectivity of adipic acid is 83%, and the NHS decomposition rate after the reaction is 6%. For the convenience of comparison, the main The reaction conditions and reaction results are listed in Table 1.
Embodiment 2
[0031] 5mol acetic acid, 0.02mol NHPI, 0.01mol cobalt acetate, 0.01mol manganese acetate, 0.01mol copper acetate, 1mol cyclohexane, 0.01mol p-tert-butylphenol, and 0.01mol triphenyl phosphite were added to a 1-liter high-pressure reactor ( Equipped with a reflux condensing device, the reflux condensing device is connected to the atmosphere through a backup pressure valve), sealed and stirred, heated to 90°C, continuously fed with air at 5 liters / min, and kept at 3MPa to control the internal pressure of the kettle. After 5 hours of reaction , cooled to room temperature, and the reaction mixture was taken out for analysis. The analysis results showed that the conversion rate of cyclohexane was 47%, the selectivity of adipic acid was 81%, and the NHS decomposition rate after the reaction was 12%. For the convenience of comparison, the main reaction conditions and reaction results are listed in Table 1.
Embodiment 3
[0033] 5mol acetic acid, 0.02mol NHS, 0.01mol cobalt acetate, 0.01mol manganese acetate, 0.01mol copper acetate, 1mol cyclohexane, 0.01mol citric acid and 0.01mol triphenyl phosphite were added to a 1-liter high-pressure reactor (with reflux The condensing device, the reflux condensing device is connected to the atmosphere through the backup pressure valve), sealed and stirred, heated to 90°C, continuously fed with air at 5 liters / min, and kept at 3MPa to control the pressure in the kettle. After 5 hours of reaction, cool To room temperature, take out the reaction mixture for analysis, analysis results: cyclohexane conversion rate 42%, adipic acid selectivity 78%, NHS decomposition rate after the reaction is 18%, for the convenience of comparison, the main reaction conditions and reaction results are listed in the table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com