Method for preparing aryl ketone based on iron-catalyzed free radical-free radical coupling reaction such as ketonic acid decarboxylation and fatty aldehyde de-carbonylation
A technology for decarboxylation of keto acids and decarbonylation of aldehydes, applied in the field of synthesis of aryl ketone derivatives, can solve the problems of stoichiometric consumption of organometallic reagents, many reaction steps, large environmental impact, etc., and achieves low cost and simple steps. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
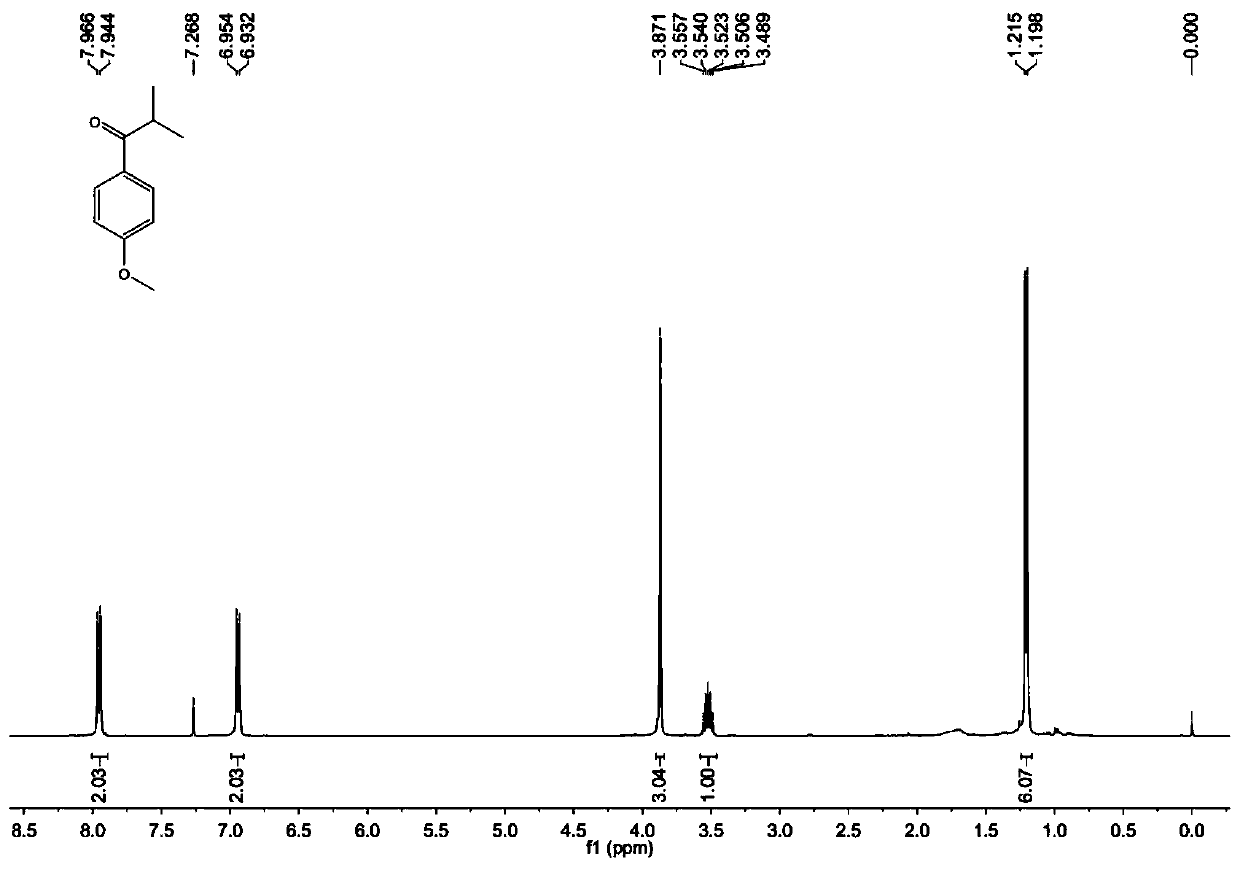
[0030] Benzoylformic acid (30.0mg, 0.2mmol), Fe(acac) 3 (21.2mg, 0.06mmol), isobutyraldehyde (72.0mg, 1.0mmol), DTBP (58.5mg, 0.4mmol), K 2 S 2 o 8 (81.1 mg, 0.3 mmol) and a stirring bar were placed in a reaction tube, and 1 mL of ethyl acetate was added as a solvent to seal the reaction tube. Put the reaction tube into an oil bath at 120° C., start stirring, and react at a constant temperature for 12 hours. After cooling the reaction mixture to room temperature, the solid residue was filtered through a short plug of silica gel and washed with 10 mL of ethyl acetate. After evaporating the solvent in vacuo, the crude product was subjected to column chromatography with petroleum ether: ethyl acetate = 30:1 as the eluent to obtain a pure product. Colorless oil, yield 74%. 1 H NMR (400MHz, CDCl 3 )δ7.96(d, J=7.6Hz, 2H), 7.57-7.54(m, 1H), 7.49-7.45(m, 2H), 3.60-3.53(m, 1H), 1.22(d, J=6.8Hz ,6H); 13 C NMR (100MHz, CDCl 3 )δ204.6, 136.2, 132.8, 128.6, 128.3, 35.4, 19.2; -1 ...
Embodiment 2
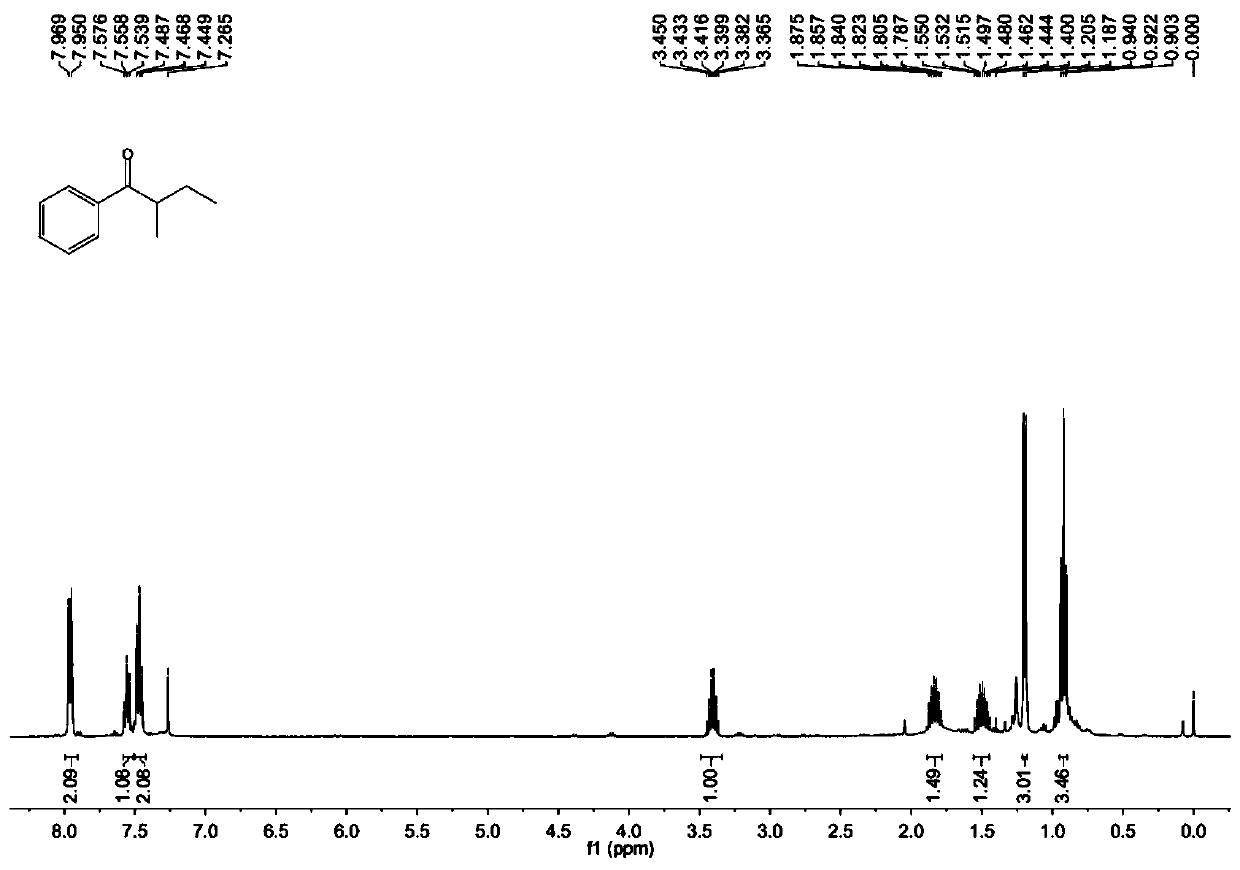
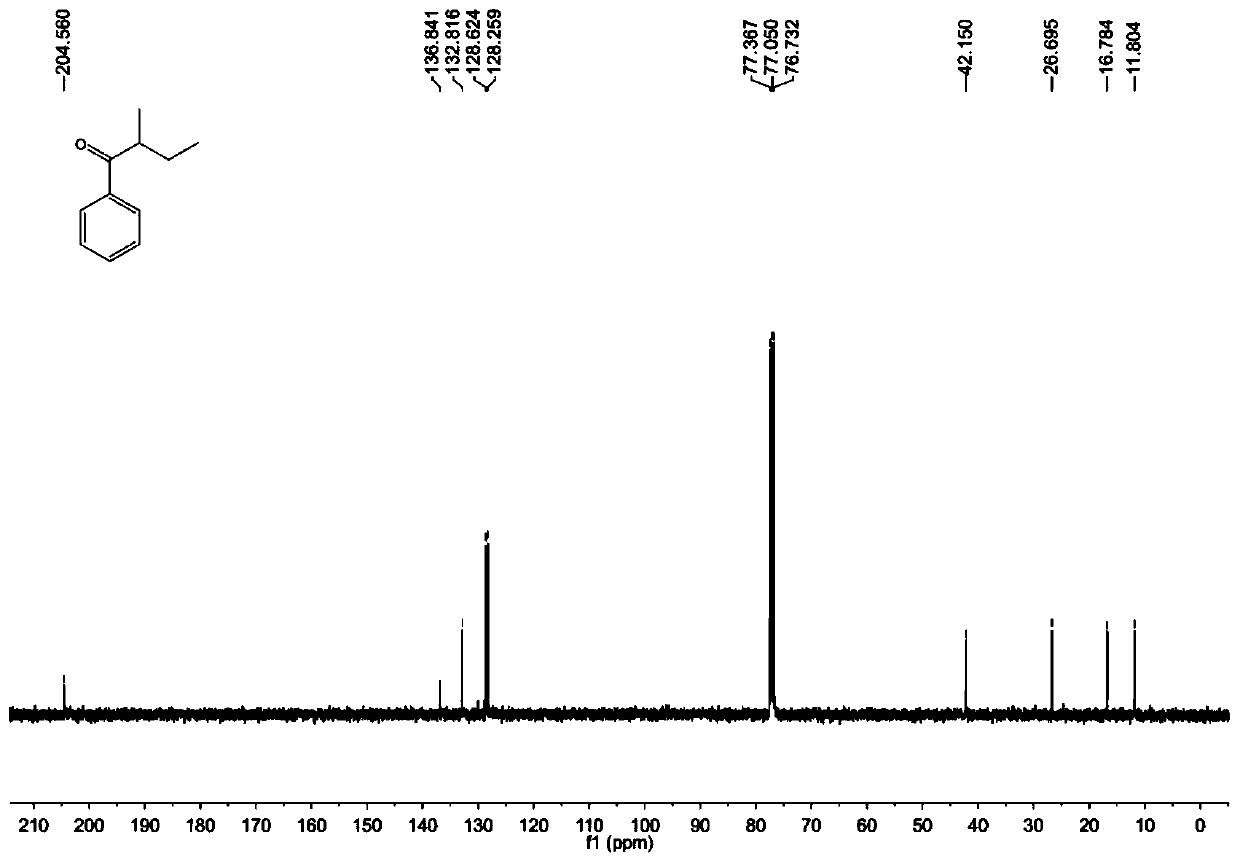
[0032] Benzoylformic acid (30.0mg, 0.2mmol), Fe(acac) 3 (21.2mg, 0.06mmol), 2-methylbutanal (86.0mg, 1.0mmol), DTBP (58.5mg, 0.4mmol), K 2 S 2 o 8 (81.1 mg, 0.3 mmol) and a stirring bar were placed in a reaction tube, and 1 mL of ethyl acetate was added as a solvent to seal the reaction tube. Put the reaction tube into an oil bath at 120° C., start stirring, and react at a constant temperature for 12 hours. After cooling the reaction mixture to room temperature, the solid residue was filtered through a short plug of silica gel and washed with 10 mL of ethyl acetate. After the solvent was evaporated in vacuo, the crude product was subjected to column chromatography with petroleum ether: ethyl acetate = 50:1 as the eluent to obtain a pure product. Yellow oil, yield 65%. 1 H NMR (400MHz, CDCl 3 )δ7.96(d, J=7.6Hz, 2H), 7.56(t, J=7.4Hz, 1H), 7.47(t, J=7.6Hz, 2H), 3.45-3.37(m, 1H), 1.88- 1.79(m,1H),1.55-1.40(m,1H),1.20(d,J=7.2Hz,3H),0.92(t,J=7.4Hz,3H); 13 C NMR (100MHz, CDCl...
Embodiment 3
[0034] Benzoylformic acid (30.0mg, 0.2mmol), Fe(acac) 3 (21.2mg, 0.06mmol), 2-methylpentanal (100.0mg, 1.0mmol), DTBP (58.5mg, 0.4mmol), K 2 S 2 o 8 (81.1 mg, 0.3 mmol) and a stirring bar were placed in a reaction tube, and 1 mL of ethyl acetate was added as a solvent to seal the reaction tube. Put the reaction tube into an oil bath at 120° C., start stirring, and react at a constant temperature for 12 hours. After cooling the reaction mixture to room temperature, the solid residue was filtered through a short plug of silica gel and washed with 10 mL of ethyl acetate. After evaporating the solvent in vacuo, the crude product was subjected to column chromatography with petroleum ether: ethyl acetate = 70:1 as the eluent to obtain a pure product. Yellow oil, yield 63%. 1 H NMR (400MHz, CDCl 3 )δ7.97-7.95(m,2H),7.56(t,J=7.1Hz,1H),7.47(t,J=7.6Hz,2H),3.43-3.45(m,1H),1.82-1.75(m ,1H),1.43-1.25(m,3H),1.19(d,J=6.8Hz,3H),0.91(t,J=7.2Hz,3H); 13 C NMR (100MHz, CDCl 3 )δ204.6, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com