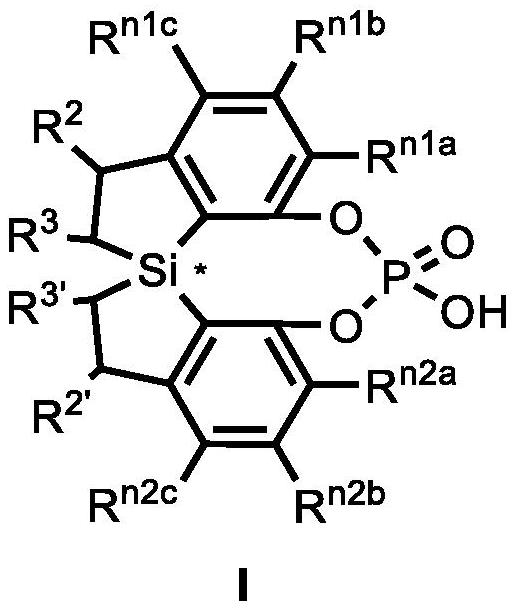
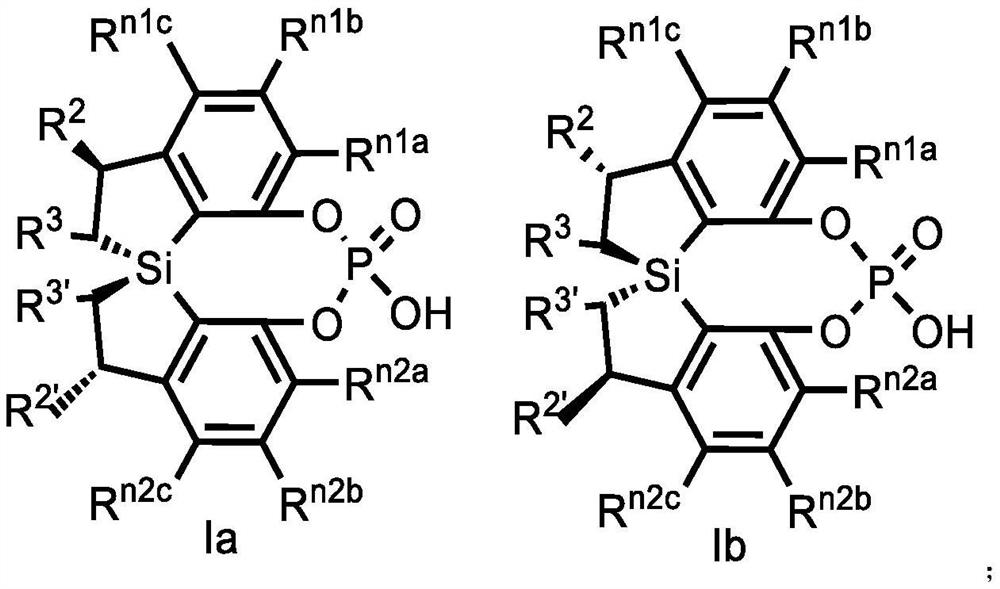
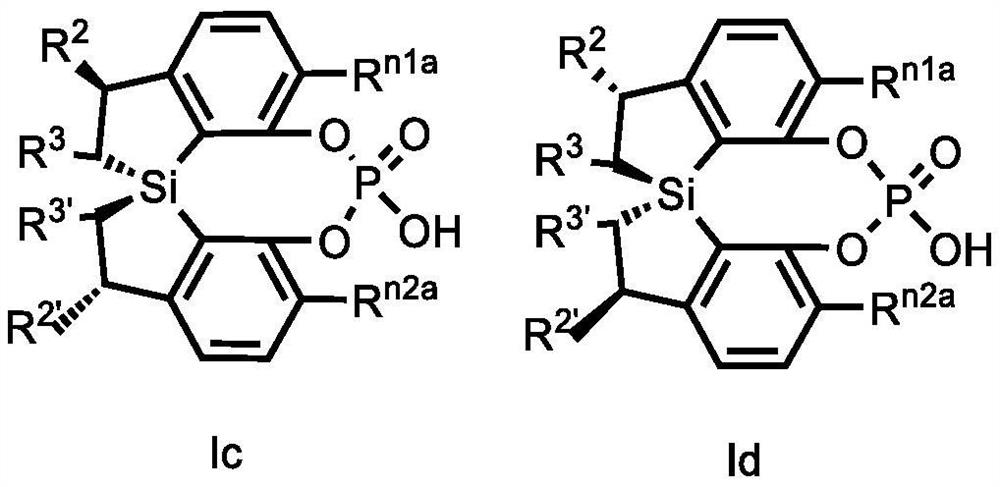
Spiro bis(dihydrobenzosilole) phosphoric acid compound as well as synthesis method and application of spiro bis(dihydrobenzosilole) phosphoric acid compound
A technology for spirobisdihydrobenzothiazole phosphoric acid and compounds, which is applied in the directions of organic chemistry methods, carbon-based compound preparation, chemical instruments and methods, etc. Mild, readily available effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0291] Embodiment 2: I-2
[0292]
[0293] White solid, 78% yield, [a] D 24 =+101.87 (c=0.90, CHCl 3 ); 1H NMR (400MHz, chloroform-d) δ7.34(t, J=7.6Hz, 2H), 7.16(d, J=7.6Hz, 2H), 7.05(d, J=8.0Hz, 2H), 3.54(p ,J=7.6Hz,2H),1.75(dd,J=14.0,8.4Hz,2H),1.45(d,J=6.8Hz,6H),1.07(d,J=14.0Hz,2H); 13 C NMR (100MHz, chloroform-d) δ162.18, 154.17, 132.78, 127.72, 122.97, 120.21, 40.12, 25.52, 5.96; 31 PNMR (162MHz, chloroform-d) δ-7.98.; HRMS (ESI-TOF) m / z calculated value C 18 h 19 o 4 SiP[M+H] + :359.0863, measured value: 359.0863.
Embodiment 3
[0294] Embodiment 3: I-3
[0295]
[0296] White solid, equivalent conversion, [a] D 24 =-185.67 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, chloroform-d) δ7.33 (s, 2H), 7.14 (d, J = 32.4Hz, 4H), 3.05–3.28 (m, 4H), 1.41–1.33 (m, 4H); 13 C NMR (101MHz, chloroform-d) δ156.70, 154.06, 132.67, 128.63, 123.36, 118.63, 31.69, 14.58; 31 P NMR (162MHz, chloroform-d) δ-7.23; HRMS (ESI-TOF) m / z calculated value C 16 h 16 o 4 SiP[M+H] + :331.0553, measured value: 331.0550.
Embodiment 4
[0297] Embodiment 4: I-4
[0298]
[0299] White solid, equivalent conversion, [a] D 24 =+185.67 (c=0.90, CHCl 3 ); 1 H NMR (400MHz, chloroform-d) δ7.33 (s, 2H), 7.14 (d, J = 32.4Hz, 4H), 3.05–3.28 (m, 4H), 1.41–1.33 (m, 4H); 13 C NMR (101MHz, chloroform-d) δ156.70, 154.06, 132.67, 128.63, 123.36, 118.63, 31.69, 14.58; 31 P NMR (162MHz, chloroform-d) δ-7.23; HRMS (ESI-TOF) m / z calculated value C 16 h 16 o 4 SiP[M+H] + :331.0553, measured value: 331.0550.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com