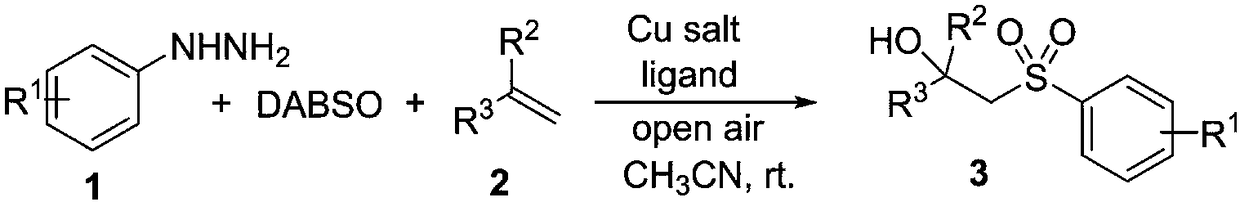
Synthesis method of beta-hydroxyl substituted sulfonyl compound
A synthesis method and compound technology, applied in the field of synthesis of sulfonyl compounds, achieving the effects of low cost, simple raw materials, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
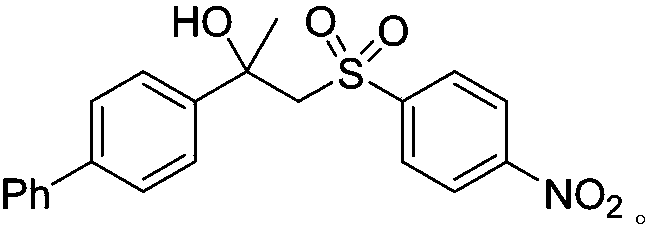
[0025] Add CuBr in turn to the reaction tube 2 (0.04mmol, 0.2 equivalent), 1,10-phenanthroline (0.04mmol, 0.2 equivalent), DABCO.(SO 2 ) 2 (0.4mmol, 2.0 equivalents), alkenes (0.2mmol, 1.0 equivalents), p-nitrophenylhydrazine (0.04mmol, 0.2 equivalents) and solvent acetonitrile (2mL), in air, stirred at room temperature for 24 hours, until TLC detected complete reaction , the reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain the corresponding β-hydroxyl-substituted sulfonyl compound 3a in a yield of 47%.
[0026] The prepared β-hydroxyl-substituted sulfonyl compound 3a has the following structural formula:
[0027]
[0028] Detected by proton nuclear magnetic resonance spectrum and carbon spectrum, the results are as follows:
[0029] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 8.11 (d, J = 8.7Hz, 2H), 7.69 (d, J = 8.7Hz, 2H), 7.44 (d, J = 4.2Hz, 4H), 7.40-7.32 (m, 3H) , 7.30-7.22 (m, 2H), 4.37 (s, 1H), 3.90 (d, J = 15.0...
Embodiment 2
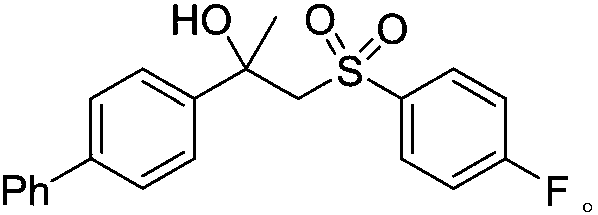
[0032] Add CuBr in turn to the reaction tube 2 (0.04mmol, 0.2 equivalent), 1,10-phenanthroline (0.04mmol, 0.2 equivalent), DABCO . (SO 2 ) 2 (0.4mmol, 2.0 equivalents), alkene (0.2mmol, 1.0 equivalents), p-fluorophenylhydrazine (0.04mmol, 0.2 equivalents) and solvent acetonitrile (2mL), in air, stirred at room temperature for 24 hours, until TLC detected complete reaction, The reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain the corresponding β-hydroxyl-substituted sulfonyl compound 3b in a yield of 61%.
[0033] The prepared β-hydroxyl-substituted sulfonyl compound 3b is as follows:
[0034]
[0035] Detected by proton nuclear magnetic resonance spectrum and fluorine spectrum, the results are as follows:
[0036] 1 H NMR (400MHz, CDCl 3): δ (ppm) 7.57-7.50 (m, 4H), 7.46 (t, J = 7.3Hz, 2H), 7.37 (t, J = 7.3Hz, 3H), 7.33-7.24 (m, 2H), 6.98 ( t,J=8.1Hz, 2H), 4.64(s,1H), 3.84(d,J=14.8Hz,1H), 3.69(d,J=14.9Hz,1H), ...
Embodiment 3
[0039] Add CuBr in turn to the reaction tube 2 (0.04mmol, 0.2 equivalent), 1,10-phenanthroline (0.04mmol, 0.2 equivalent), DABCO.(SO 2 ) 2 (0.4mmol, 2.0 equivalent), solvent acetonitrile (2mL), and then inject olefin (0.2mmol, 1.0 equivalent) and phenylhydrazine (0.04mmol, 0.2 equivalent) with a micro-syringe, stir at room temperature for 24 hours in air, until TLC detection After complete reaction, the reaction solution was concentrated under reduced pressure and separated by column chromatography to obtain the corresponding β-hydroxyl-substituted sulfonyl compound 3c in a yield of 46%.
[0040] The prepared β-hydroxyl-substituted sulfonyl compound 3c is as follows:
[0041]
[0042] Detected by proton nuclear magnetic resonance spectrum and carbon spectrum, the results are as follows:
[0043] 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 7.63 (d, J = 7.4Hz, 2H), 7.56 (t, J = 7.4Hz, 1H), 7.42 (t, J = 7.5Hz, 2H), 6.77 (t, J = 10.9Hz ,1H),6.68(s,1H),6.61(d,J=8.1Hz,1H),5.88(d,J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com