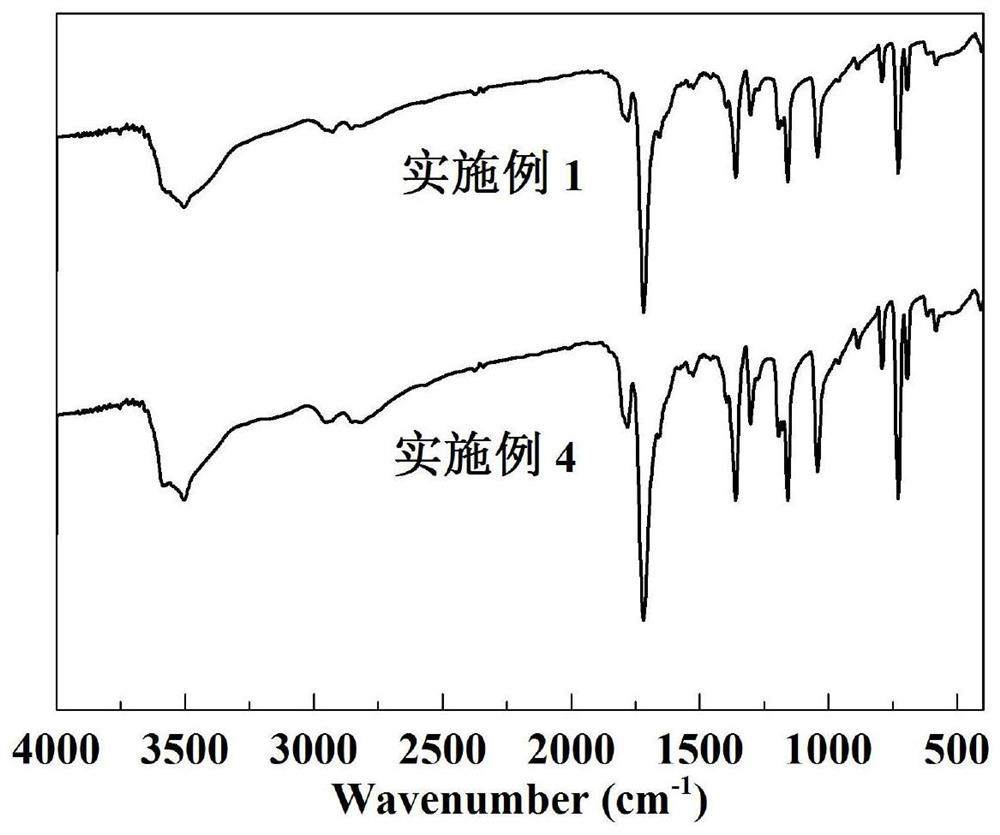
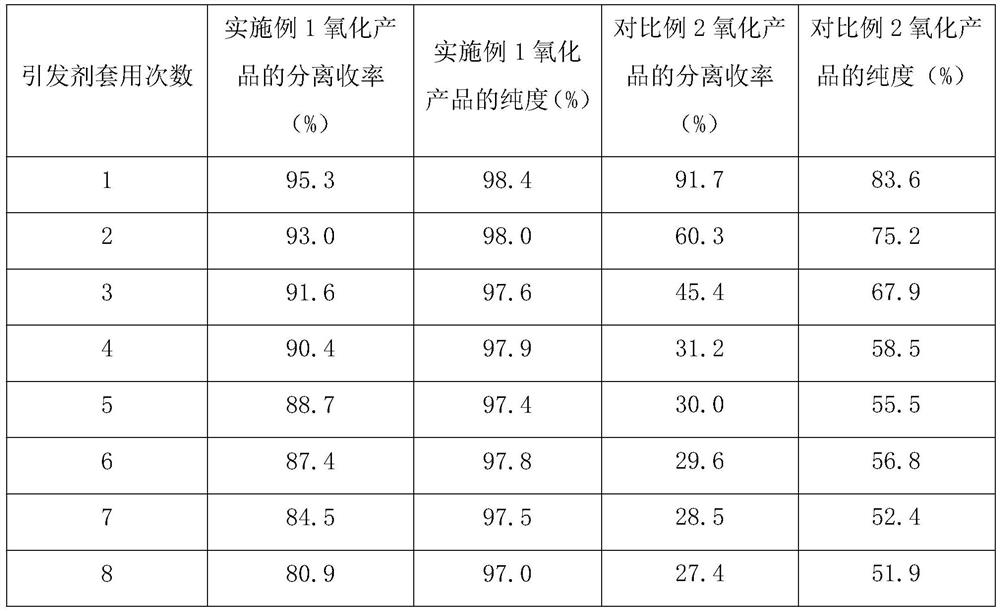
Preparation method of free radical initiator and application of free radical initiator in oxidation reaction
An oxidation reaction and initiator technology, which is applied in the field of free radical initiator preparation, can solve the problems of refractory organic compounds, high N/C, stimulation, etc., and achieve good preparation repeatability, high separation yield and purity, and raw materials simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Under the condition of an ice-water bath, add 2.2g of hydroxylamine sulfate, 2.2g of anhydrous sodium carbonate, and 150mL of water into the flask, stir and dissolve, react for 1.5h, then add 6.0g of tetrachlorophthalic anhydride, and stir for 1.0h at room temperature , then raise the temperature to 85°C, and continue the reaction for 12h. After the reaction, cool, filter, wash with water, and dry at 60°C for 12h, then use a mixed solvent of toluene and ethanol (1:1) to recrystallize, filter, and After drying for 12 hours, light brown TCNHPI was obtained, with a melting point of 238°C, a yield of 90.4%, and a purity of 99.1%.
[0029] The TCNHPI prepared in this embodiment is used as an initiator, the above-mentioned loaded multi-metal oxide is used as a catalyst, and glacial acetic acid is used as a solvent to evaluate its catalyzed recycling performance of molecular oxygen oxidation of 2-chloro-4-thiamphenicol toluene, specifically Proceed as follows:
[0030] Put 20...
Embodiment 2
[0032]Under the condition of ice-water bath, add 2.2g of hydroxylamine sulfate, 1.6g of anhydrous sodium carbonate, and 240mL of water into the flask, stir and dissolve, react for 3.0h, then add 6.0g of tetrachlorophthalic anhydride, and stir for 2.0h at room temperature , then raise the temperature to 90°C, and continue the reaction for 8h. After the reaction, cool, filter, wash with water, and dry at 60°C for 12h, then recrystallize with a mixed solvent of toluene and ethanol (1:1), filter, and dry at 60°C After drying for 24 hours, light brown TCNHPI was obtained, with a melting point of 239°C, a yield of 89.0%, and a purity of 99.4%.
[0033] The TCNHPI prepared in this embodiment is used as an initiator, the above-mentioned loaded multi-metal oxide is used as a catalyst, and glacial acetic acid is used as a solvent to evaluate its catalyzed recycling performance of molecular oxygen oxidation of 2-chloro-4-thiamphenicol toluene, specifically Proceed as follows:
[0034] P...
Embodiment 3
[0036] Under the condition of an ice-water bath, add 1.9g of hydroxylamine sulfate, 1.7g of anhydrous sodium carbonate, and 180mL of water into the flask and stir to dissolve, react for 2.0h, then add 6.0g of tetrachlorophthalic anhydride, and stir for 4.0h at room temperature , then raise the temperature to 75°C, and continue the reaction for 16h. After the reaction, cool, filter, wash with water, and dry at 60°C for 12h, then use a mixed solvent of toluene and ethanol (1:1) to recrystallize, filter, and dry After drying for 12 hours, light brown TCNHPI was obtained, with a melting point of 237°C, a yield of 89.6%, and a purity of 98.8%.
[0037] The TCNHPI prepared in this embodiment is used as an initiator, the above-mentioned loaded multi-metal oxide is used as a catalyst, and glacial acetic acid is used as a solvent to evaluate its catalyzed recycling performance of molecular oxygen oxidation of 2-chloro-4-thiamphenicol toluene, specifically Proceed as follows:
[0038] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com