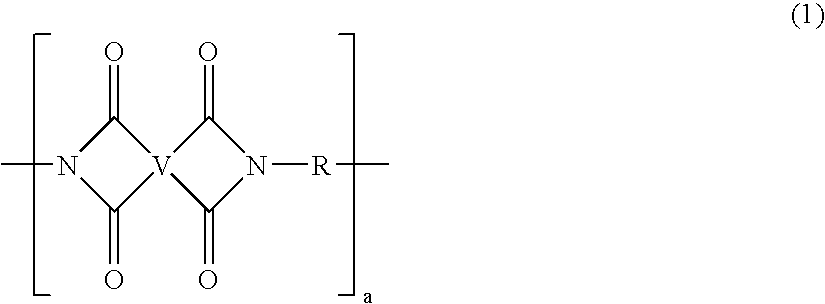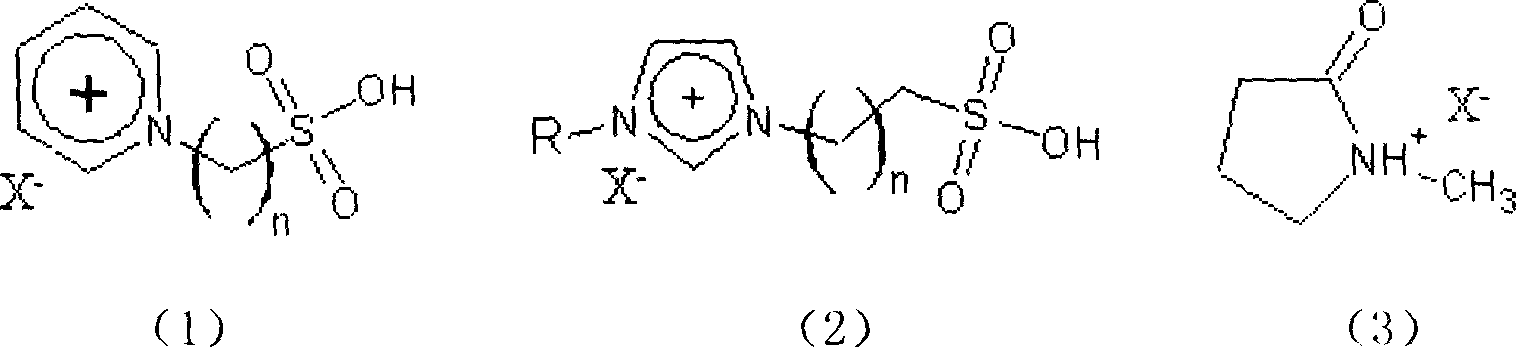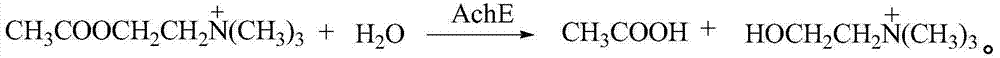Patents
Literature
373 results about "Phthalic acid anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of manufacture of bis(phthalimide)s and polyetherimides, and bis(phthalimide)s, and polyetherimides formed therefrom
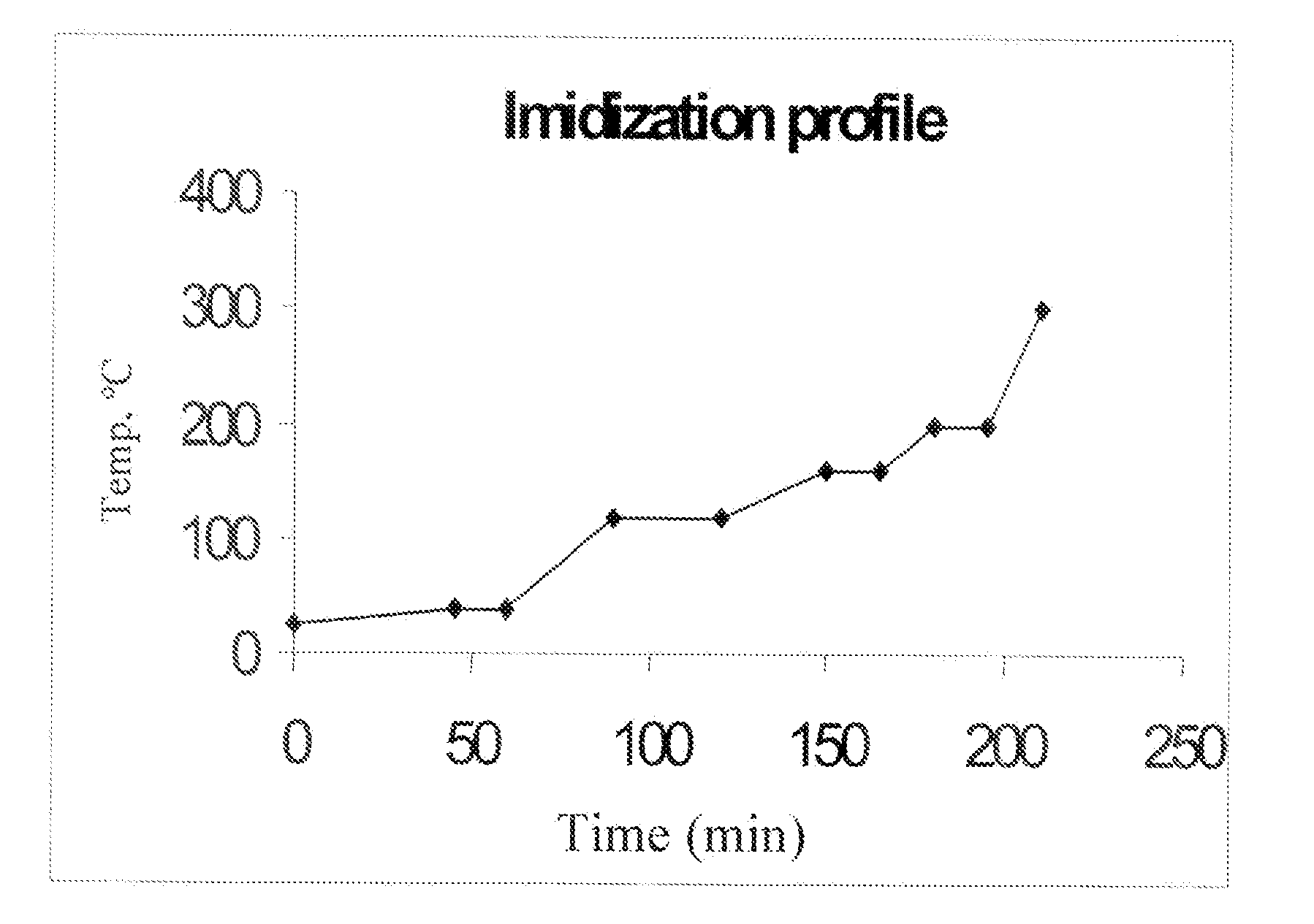
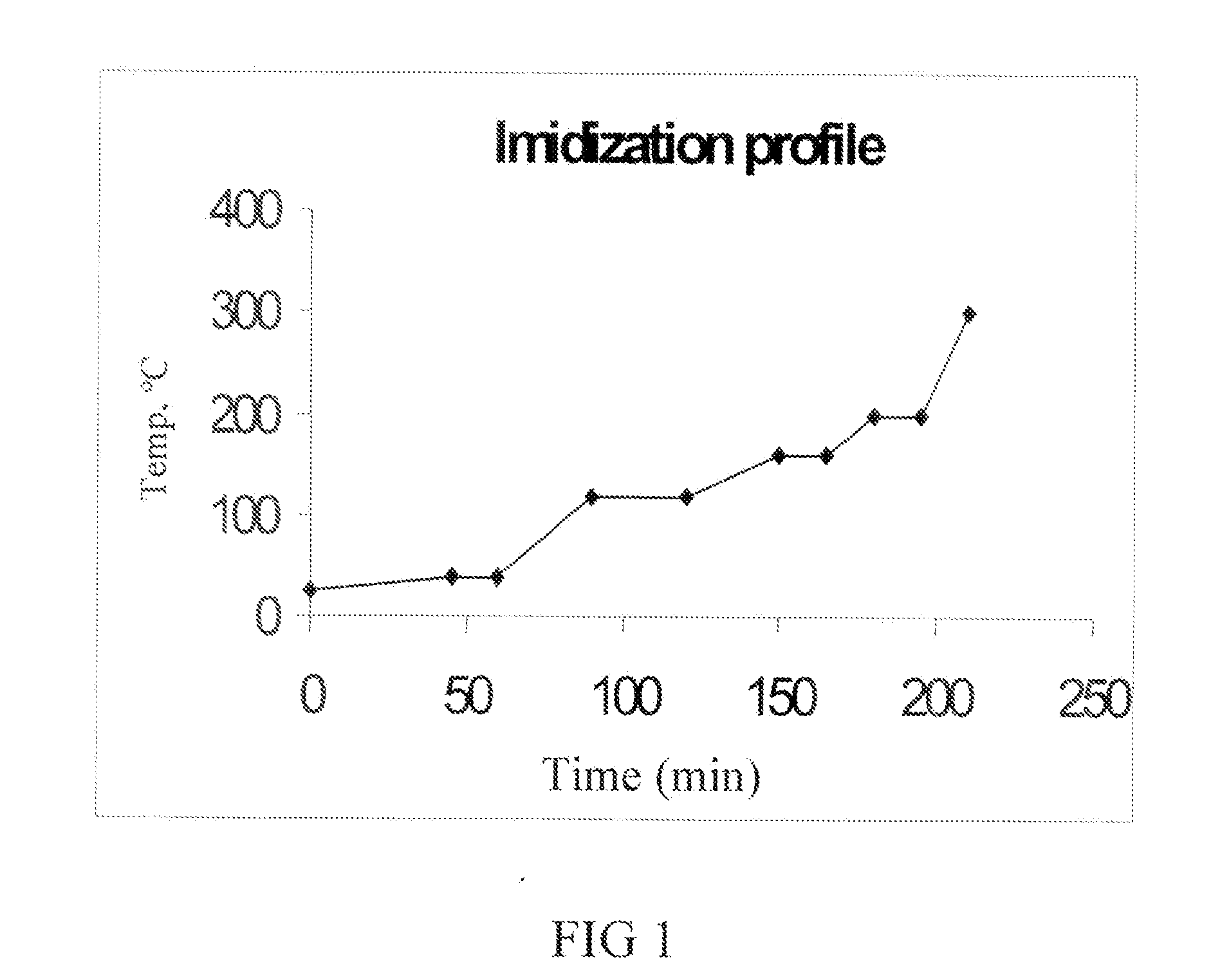
A method of manufacture of a bis(phthalimide) composition includes reacting, in the presence of a solvent and a catalytically active amount of an imidization catalyst selected from quaternary ammonium salts, quaternary phosphonium salts, and combinations thereof, a substituted phthalic anhydride with an organic diamine, wherein conversion to the bis(phthalimide) is 99% complete in less than 6 hours.
Owner:SABIC GLOBAL TECH BV
Hot melt adhesive composition
A hot melt adhesive composition comprising: (a) a functional copolymer obtained from copolymerization of ethylene and a comonomer selected from maleic anhydride, citraconic anhydride, itaconic anhydride, tetrahydrophthalic anhydride, maleic acid, citraconic acid, itaconic acid, fumaric acid, tetrahydrophthalic acid, the corresponding salts, monoesters and diesters of these acids, and mixtures of any of these, wherein the functional copolymer is present in an amount from about 5 to about 95 weight % of the combined total amount of (a) and (b); and (b) at least one ethylene copolymer obtained from copolymerization of ethylene with a polar monomer wherein said polar comonomer is present in the copolymer in an amount of from 8 to 40 weight %, wherein said ethylene copolymer is selected from the group consisting of ethylene / vinyl acetate copolymers, ethylene / alkyl (meth)acrylate copolymers and ethylene / alkyl (meth)acrylate / carbon monoxide terpolymers, in an amount from about 5 to about 95 weight % of the combined total amount of (a) and (b); wherein the composition has a melt index of 100 grams / 10 minutes or higher. Articles comprising and process using the hot melt adhesive are also disclosed.
Owner:DOW GLOBAL TECH LLC
Articles comprising a polyimide solvent cast film having a low coefficient of thermal expansion and method of manufacture thereof
ActiveUS20080044682A1Printed circuit aspectsSynthetic resin layered productsPolymer sciencePolyetherimide
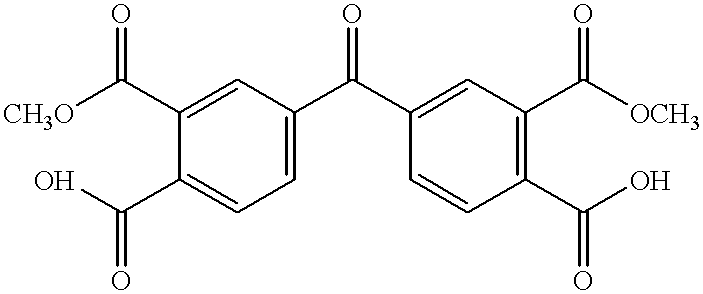
An article comprises a solvent cast film comprising a polyetherimide comprising structural units derived from a dianhydride component selected from the group consisting of 3,4′-oxydiphthalic anhydride, 3,3′-oxydiphthalic anhydride, 4,4′-oxydiphthalic anhydride, and combinations thereof, and a diamine component. The polyetherimide has a glass transition temperature that is at least 190° C. The film has a coefficient of thermal expansion of less than 60 ppm / ° C., a thickness from 0.1 to 250 micrometers, and less than 5% residual solvent by weight. The film has less than 15 molar % of a member selected from the group consisting of biphenyltetracarboxylic acid, dianhydrides of biphenyltetracarboxylic acid, esters of biphenyltetracarboxylic acid, and combinations thereof.
Owner:SHPP GLOBAL TECH BV
Triamine-modified polymides having improved processability and low melt flow viscocity
Addition-cured polyimides that contain the reaction product of an aromatic triamine or trianhydride analogue thereof, a reactive end group such as 5-norbornene-2, 3-dicarboxylic acid, ester derivatives of 5-norbornene-2,3-dicarboxylic acid, anhydride derivatives of 5-norbornene-2,3-dicarboxylic acid, or 4-phenylethynylphthalic anhydride, an aromatic diamine, and a dialkyl ester of an aromatic tetracarboxylic acid. The resultant starlike polyimides exhibit lower melt flow viscosity than its linear counterparts, providing for improved processability of the polyimide. Also disclosed are methods for the synthesis of these polyimides as well as composite structures formed using these polyimides.
Owner:NASA
Preparation method for biological agent for preventing and controlling human papilloma virus infection
ActiveCN102631666AImprove stabilityLow costPeptide/protein ingredientsAntiviralsProtein solutionHuman papilloma virus infection
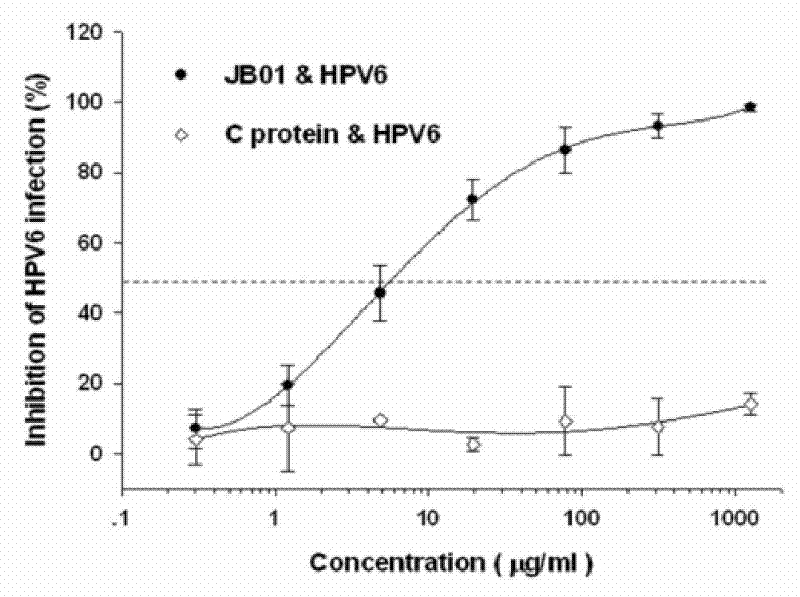
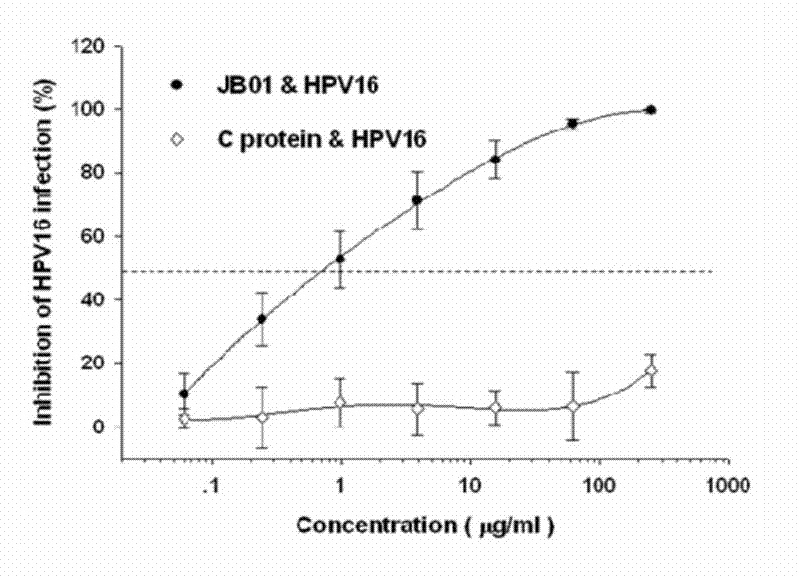
The invention discloses a preparation method for a biological agent for preventing and controlling human papilloma virus infection. The preparation method includes following steps: dissolving 3-hydroxy-phthalic anhydride HP into dimethyl sulfoxide DMSO to obtain saturated HP solution; dissolving beta-lactoglobulin beta-LG into 0.1M of sodium phosphate solution with pH (potential of hydrogen) of 8.5 to obtain protein solution with protein final concentration of 20mg / mL; and equally dividing the HP solution into five parts, adding the HP solution into the protein solution at every 12 minutes, shaking and mixing, adjusting pH of IMNaOH to be 8.5, leading the final concentration of anhydride in a reaction system to be 60mM, standing for 1 hour at the temperature of 25 DEG C, dialyzing by PBS (phosphate buffer solution) with pH of 7.4, performing filtration sterilization by a 0.45uM microfiltration membrane, and storing at the temperature of 4 DEG C so as to obtain a finished product. The biological agent has the advantages of capabilities of stopping HPV (human papilloma virus) from invading cells and stopping virus infection from spreading, safety, stability, low cost and the like.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride
ActiveCN101696199AOvercoming the Factors Affecting Industrial Safety ProductionHigh yieldOrganic chemistryAlkyl transferO-Xylene
The invention discloses a preparation method of 4,4'-(Hexafluoroisopropylidene) diphthalic anhydride (6-FDA), belonging to the technical field of liquid crystal materials. The main technical scheme is achieved as follows: o-xylene and 2,2-dichloro-hexafluoropropane undergo alkylation reaction in an ionic liquid under the catalytic action of Lewis acids (AlCl3, ZnCl2) to obtain 4,4'-(Hexafluoroisopropylidene) di(2-xylene); then the 4,4'-(Hexafluoroisopropylidene) di(2-xylene) is oxidized by permanganic acid TEBA triethylbenzylammonium salts to obtain 4,4'-(Hexafluoroisopropylidene) diphthalandione; and finally, the 4,4'-(Hexafluoroisopropylidene) diphthalandione is dehydrated by acetic oxide to obtain the 4,4'-(Hexafluoroisopropylidene) diphthalicanhydride (6-FDA). The preparation method has the advantages of mild reaction condition, less three-waste emission and high yield.
Owner:天津众泰材料科技有限公司
Cold-resistant and high-temperature resistant cable material and preparation method thereof
ActiveCN104017333AGood cold and high temperature resistanceLess affected by extreme temperature conditionsEpoxyPolymer science
The invention belongs to the field of a cable, and discloses a cold-resistant and high-temperature resistant cable material. The cold-resistant and high-temperature resistant cable material is prepared from the following raw materials in parts by weight: 20-22 parts of dicyclopentadiene phenol epoxy resin, 15-16 parts of polypropylene resin, 6-8 parts of gamma-aminopropyltriethoxysilane, 5-6 parts of phthalic anhydride, 5-6 parts of alpha-methacrylic acid, 3-4 parts of modified kaolinite, 3-4 parts of modified kieselguhr, 2-3 parts of dimethyaminoethoxyethanol, 2-3 parts of glass fiber, 1-2 parts of polyethylene glycol, 1-2 parts of polyglycolic acid, 1-2 parts of triallyl isocyanurate, 1-2 parts of aluminium hydroxide, 1-2 parts of antimonous oxide and 1-2 parts of titanium carbide. The invention also discloses a preparation method of the cable material. The cable material prepared by the method is good in cold resistance and high-temperature resistance, and wide in application field.
Owner:东莞市台胜五金制品有限公司
Gas-phase oxidization process and process for the preparation of phthalic anhydride
InactiveUS6369240B1Change activityHigh activityOrganic oxidationOrganic compound preparationMaleic anhydrideChemistry
This invention relates to the vapor-phase oxidation of hydrocarbons by passing a gaseous mixture comprising of a molecular oxygen-containing gas and hydrocarbons which may contain substituents to a fixed bed of catalyst and provides a process for vapor-phase oxidation to be effected by passing a gaseous mixture of raw materials to a fixed bed of catalyst in which the void ratio of the catalyst layers increases by stages in one step or more from upstream downward in the flow of the gaseous mixture of raw materials. For example, the process can oxidize in vapor phase such hydrocarbons as naphthalene, xylene, benzene, toluene, durene, butene, acenaphthene, anthracene, indene and their derivatives in high yields with high productivity. Moreover, the process can prepare phthalic anhydride in high yields with high productivity by the vapor-phase oxidation of naphthalene or ortho-xylene.
Owner:NIPPON MICROMETAL
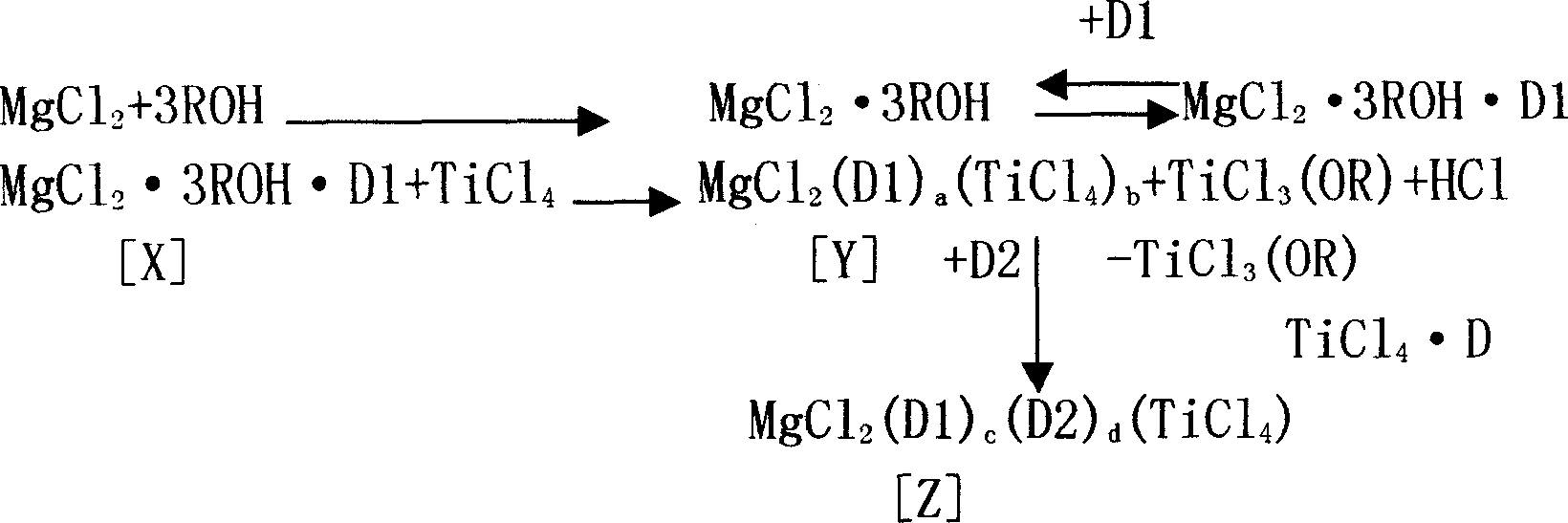
Catalyst for propylene polymerization and its preparation method
The present invention relates to catalyst, and is especially one kind of catalyst for propylene polymerization and its preparation process. The catalyst materials include MgCl2, solvent oil, isooctanol, titanate, acid anhydride, TiCl4, diisobutyl phthalate, toluene and hexane in certain molar proportion. The preparation process of the catalyst includes the following steps: 1. adding anhydrous MgCl2, No. 200 solvent oil and isooctanol into reaction bottle; stirring and heating; and adding tetrabutyl titanate and phthalic anhydride to prepare homogeneous solution; and 2. dropping the solution into glass reactor holding mixture of TiCl4 and refined toluene and adding diisobutyl phthalate.
Owner:LIAONING DINGJIDE PETROCHEM
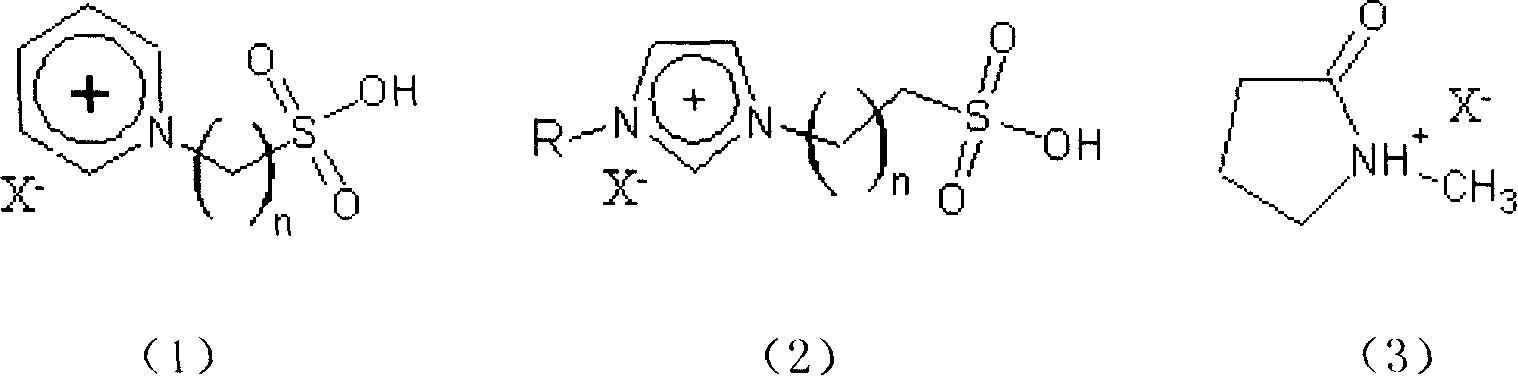
Clean esterification method for producing bialkyl ortho phthalate
InactiveCN1903824AHigh esterification capacityLow reaction temperatureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIonAcid catalyzed
The present invention relates to a clean esterification method for producing dialkyl phthalate. It is characterized by that it adopts the ionic liquid formed from sulfonic acid group functionalized alkyl pyridine cation or sulfonic acid group functionalized 1,3-dialkyl iminazol cation or 2-oxopyrrolidine cation and organic or inorganic anion X as catalyst of reaction and reaction medium; at normal pressure its reaction temperature is 90-130deg.C and reaction time is 1-2hr, it can catalyze the esterification reaction of phthalic anhydride and isooctanol or n-octanol so as to obtain the invented dialkyl phthalate.
Owner:QINGDAO UNIV OF SCI & TECH
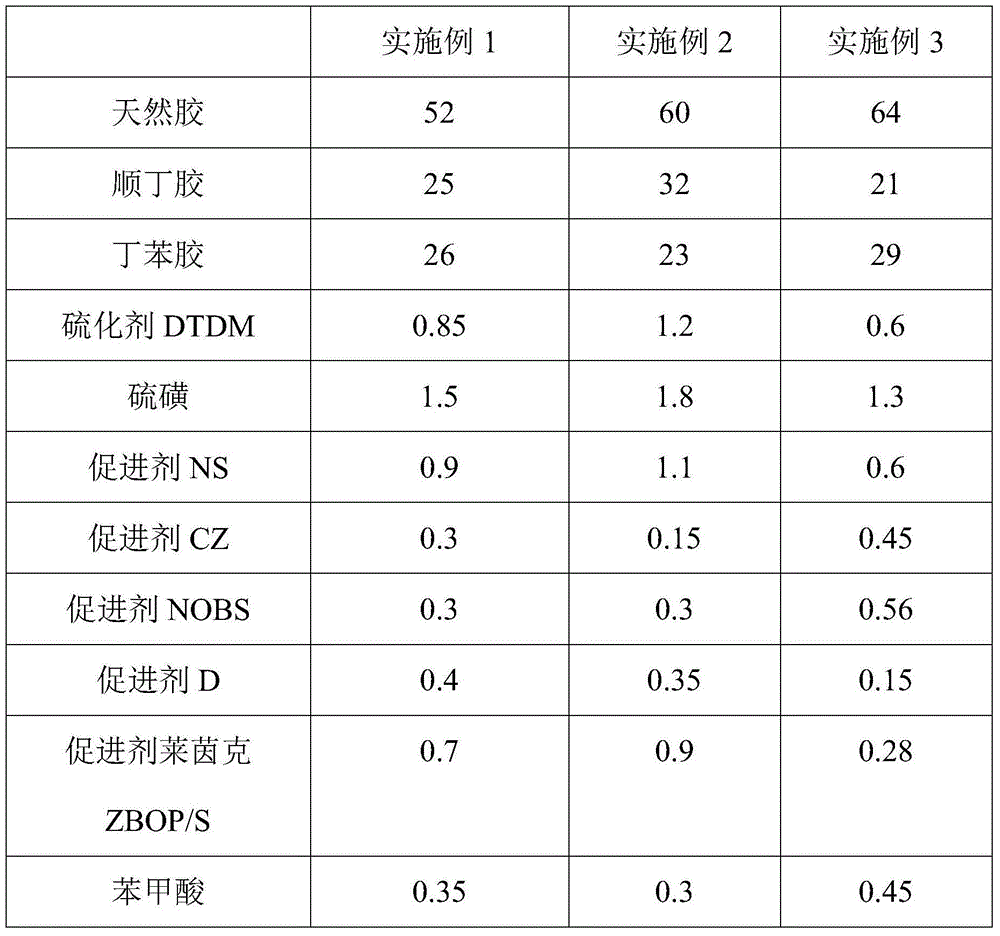
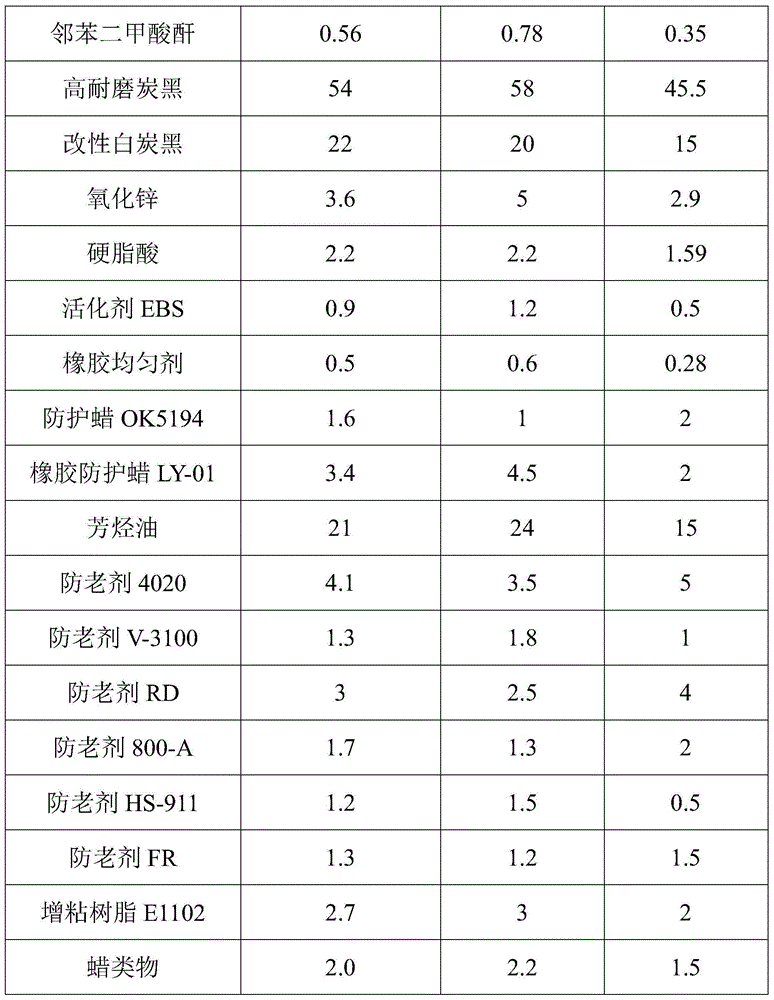
Preparation method of rubber composition used for automobile and preparation method thereof
The invention discloses a rubber composition used for automobile, which comprises the following raw materials: natural rubber, cis-1,4-polybutadiene rubber, butadiene-styrene rubber, a vulcanizing agent DTDM, sulphur, a promoter NS, a promoter CZ, a promoter NOBS, a promoter D, a promoter Rheineck ZBOP / S, benzoic acid, phthalic anhydride, high wear resistant carbon black, modified white carbon black, zinc oxide, stearic acid, an activator EBS, a rubber homogenizing agent, a protection wax OK5194, a rubber protection wax LY-01, aromatic hydrocarbons oil, an antioxidant 4020, an antioxidant V-3100, an antioxidant RD, an antioxidant 800-A, an antioxidant HS-911, an antioxidant FR, a tackifying resin E1102, and wax objects. The rubber composition has good wear resistance and heat resistance, so that the rubber composition with good comprehensive performance used for the automobile is obtained.
Owner:TONGLING SANSHENG ELECTRONICS
Specific fluorescent probe for identifying hydrazine and application thereof
InactiveCN103923071AStrong specificityGood fluorescence propertiesOrganic chemistryFluorescence/phosphorescenceChemical synthesisSodium acetate
The invention discloses a specific fluorescent probe for identifying hydrazine and an application thereof, belonging to the field of fine chemical engineering. The fluorescent probe is a 4-trifluoromethyl-7-aminocoumarin derivative, and is prepared by steps of putting 4-trifluoromethyl-7-aminocoumarin, phthalic anhydride and sodium acetate into a reaction bottle in proportion, and performing backflow by adding acetic acid. The fluorescent probe and the corresponding hydrazine content detection process cannot be interfered by biological system matrixes and impurities, so the fluorescent probe can be used for quantitative measurement of the hydrazine content in various biological systems. The probe has high specificity, and can be subjected to hydrolysis after specifically reacting with hydrazine; a hydrolysate has good fluorescence characteristics. Raw materials are cheap and easily available, can be obtained through chemical synthesis, and synthesis process is simple and feasible; the probe has high sensitivity, is suitable for detecting the hydrazine content in cells, and can be used for measuring the hydrazine content by drawing a standard curve. The probe is a ratio type probe, and effectively avoids the influences of non-uniform probe distribution, environmental factors, actuators and the like on the measurement results.
Owner:CHANGSHU RES INST OF DALIAN UNIV OF TECH CO LTD
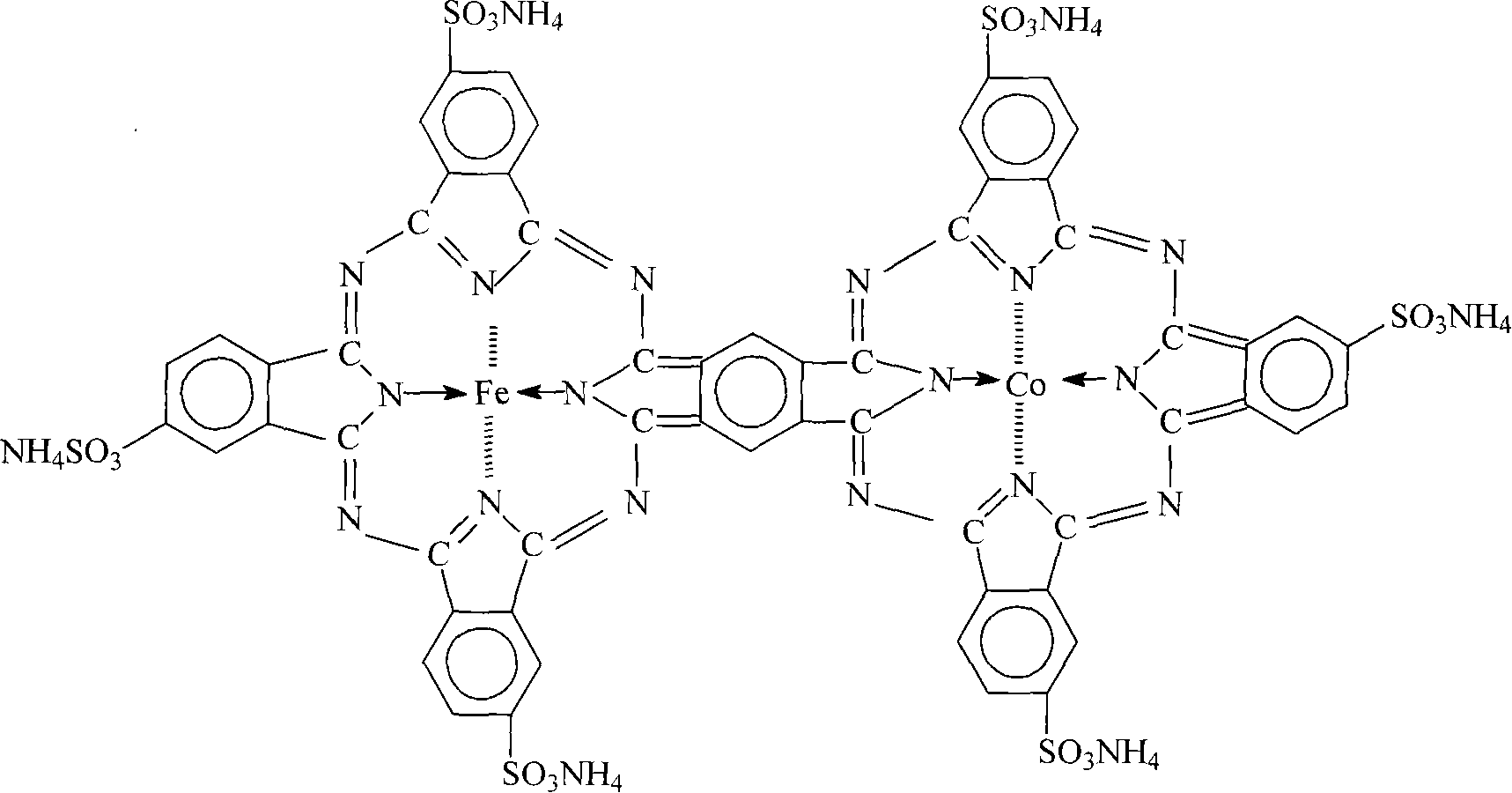
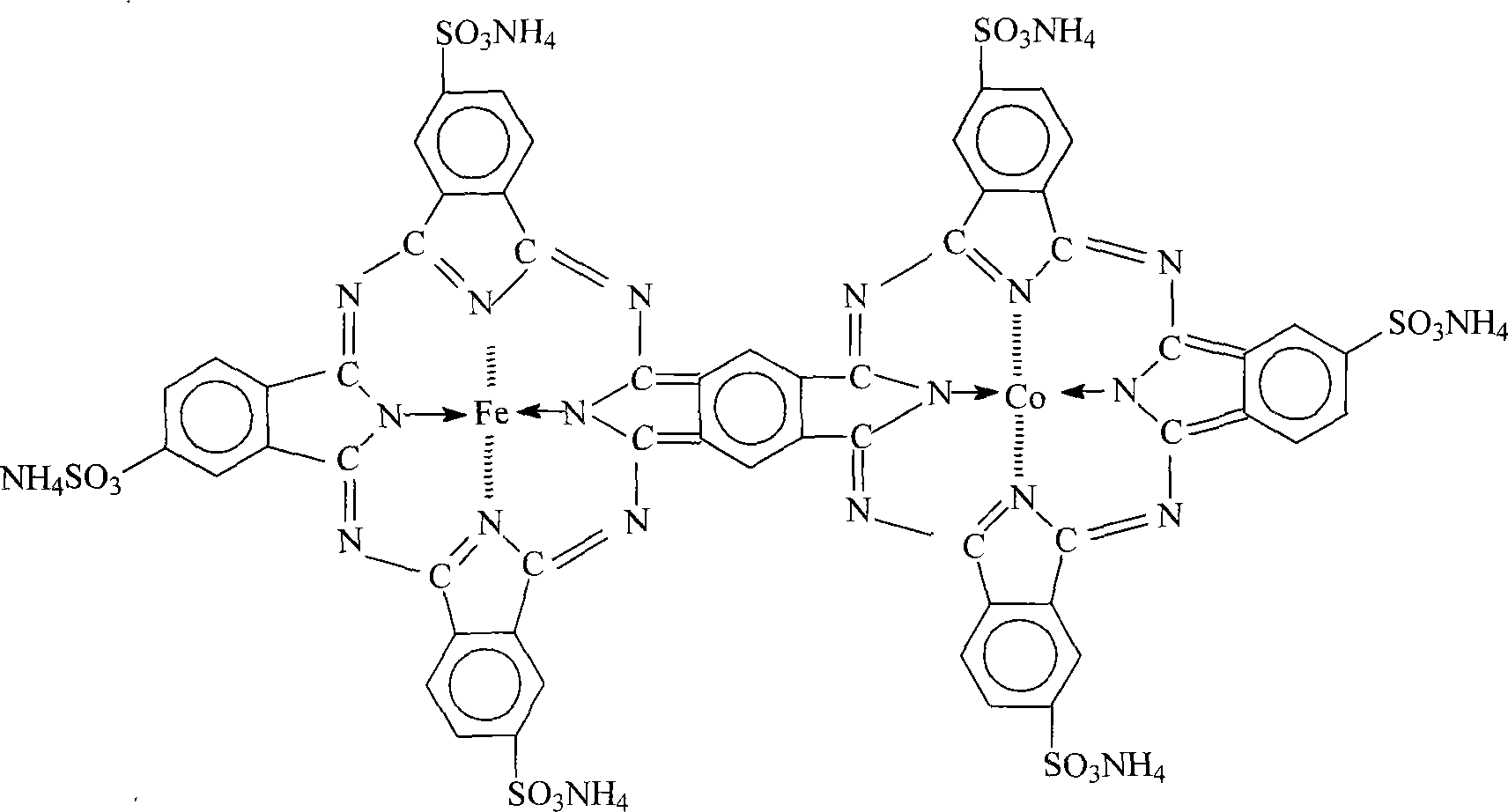
Desulphurization catalyst of sulfosalt of phthalocyanine iron cobalt, and preparation method
InactiveCN101049576AHigh catalytic activityImprove desulfurization efficiencyOrganic-compounds/hydrides/coordination-complexes catalystsDispersed particle separationIron saltsPhthalocyanine
A phthalocyanin-iron-cobalt-ammonium sulfonate as the dsulfurizing catalyst with high catalytic activity and desulfurizing efficiency (more than 99%) is prepared through preparing the sulfonated chemical from phthalic anhydride as raw material and smoking sulfuric acid as sulfonating agent, preparing phthalocyanin-iron-cobalt- ammonium sulfonate from said sulfonated chemical, ammonium molybdate as catalyst, urea, cobalt chloride and iron salt, drying, and pulverizing.
Owner:汪晓梅
Preparation method of polymeric-microsphere-carrier immobilized N-hydroxyphthalimide catalyst
InactiveCN104069891AHigh degree of immobilizationEasy to separateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemistryPhthalic acid anhydride
The invention belongs to the technical field of immobilized N-hydroxyphthalimide catalysts, and specifically relates to a preparation method of polymeric-microsphere-carrier immobilized N-hydroxyphthalimide catalyst. The preparation method is characterized in that intermediate product aldehyde group modified microsphere GMA / MMA-AL, modified microsphere GMA / MMA-PA bonded with a phthalic acid group and microsphere GMA / MMA-PPA loaded with phthalic anhydride PPA are prepared, and finally a functional microsphere loaded with N-hydroxyphthalimide functional microsphere group is obtained. With the preparation method provided by the invention, the efficient organic catalyst N-hydroxyphthalimide (NHPI) is easy to separate and can be reused, a system is easy to purity, and therefore, environment-friendly catalytic oxidation is realized.
Owner:ZHONGBEI UNIV
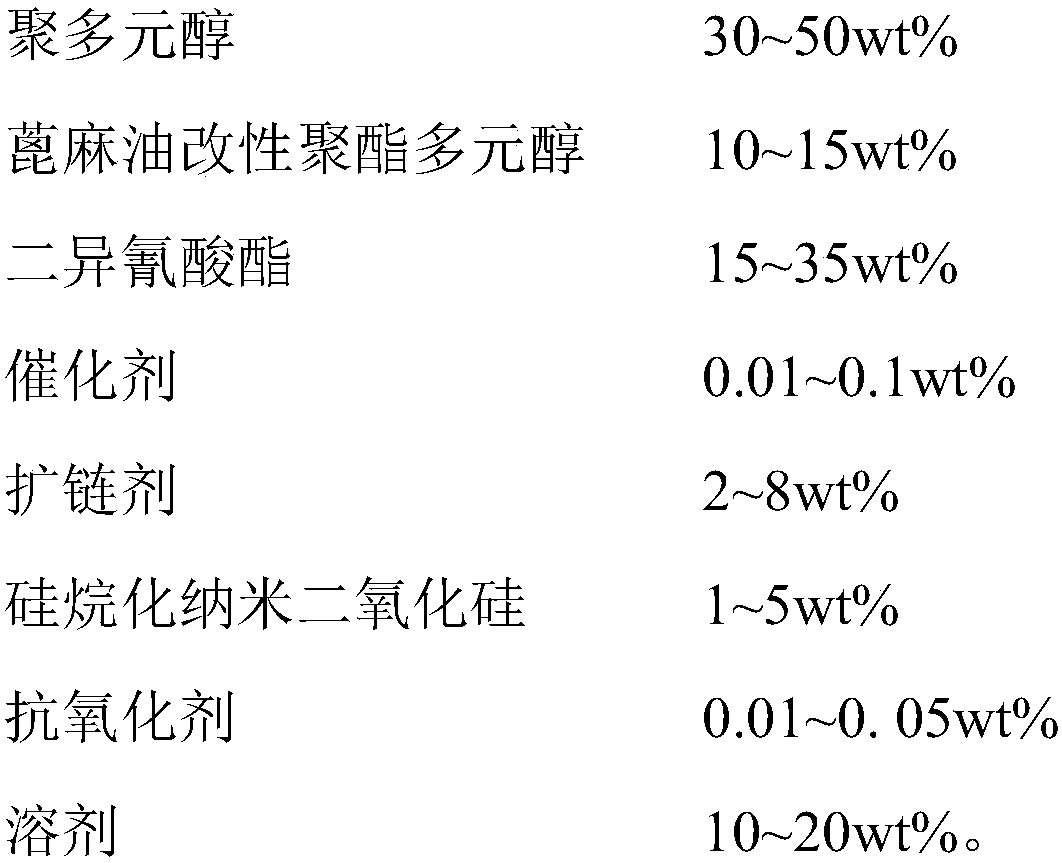
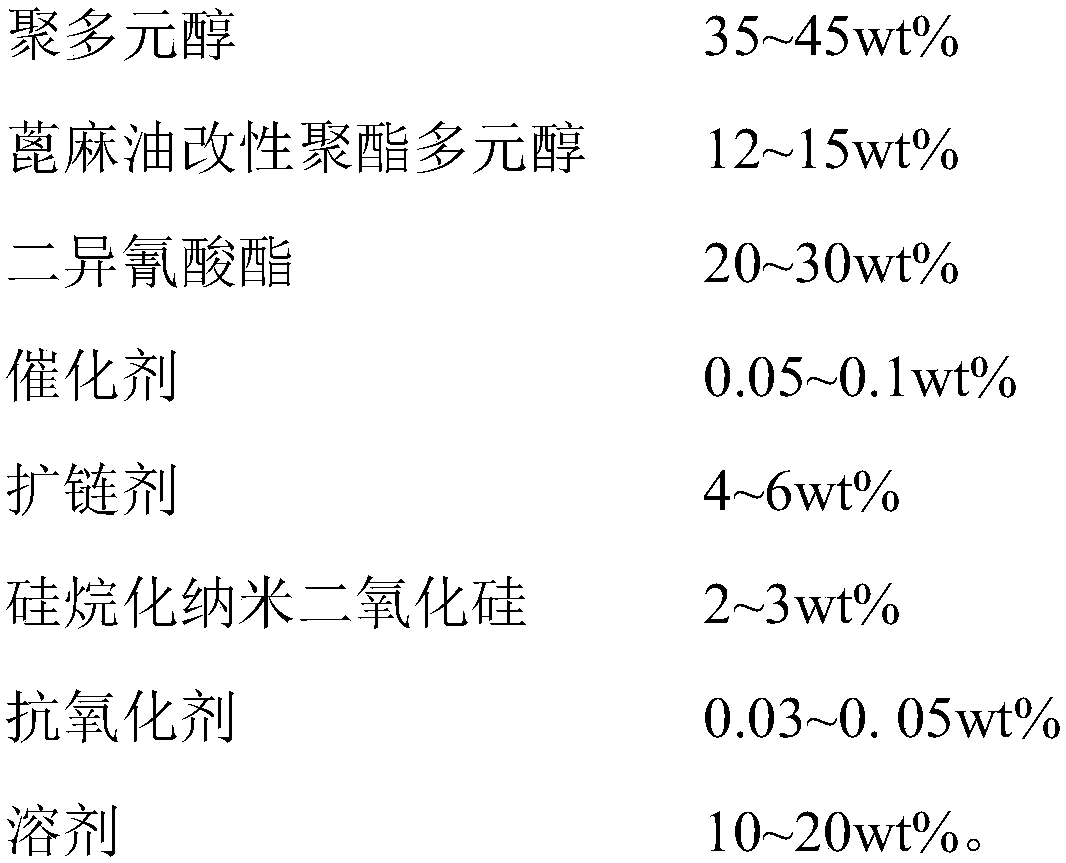
Heat-resistant single-component moisture-curing polyurethane adhesive and preparation method and application thereof
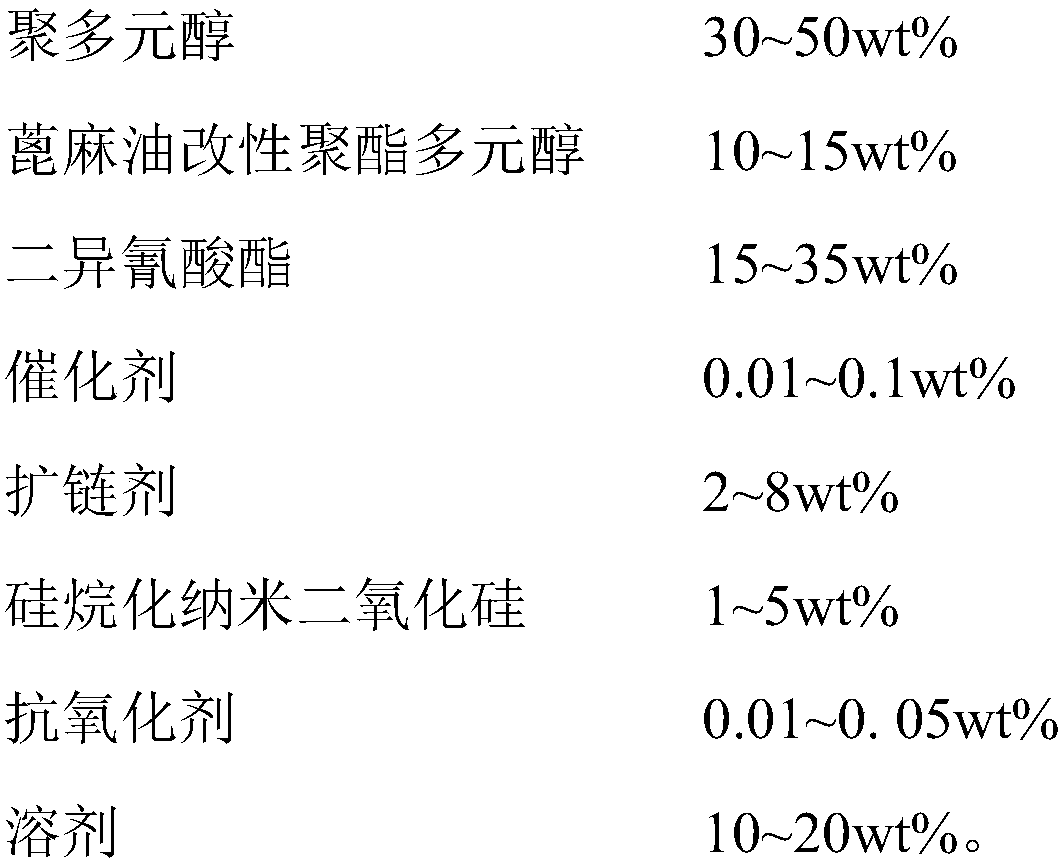
InactiveCN109749692AHigh bonding strengthImprove heat resistanceNon-macromolecular adhesive additivesPolyureas/polyurethane adhesivesPolyesterPolyurethane adhesive
The invention discloses a heat-resistant single-component moisture-curing polyurethane adhesive and a preparation method and application thereof. The preparation method comprises the following step: carrying out esterification reaction on castor oil, phthalic anhydride and dihydric alcohol at first to prepare a castor oil modified polyester polyol with the functionality of 2.0-2.5 and containing abenzene ring aromatic structure and a long fatty acid molecular segment. When partially substituted polyols are used for modification of polyurethane, rigid benzene rings can be introduced into a soft segment. Distribution of microcrystals in the soft segment structure is controlled, so that the flexibility of an adhesive layer is enhanced; the cohesive strength is increased, and the heat resistance is improved; meanwhile, the modified castor oil polyester polyol can effectively control the crosslinking degree of the polymer and prevent the drastic reduction of performance of an adhesive or coagulation caused by excessive crosslinking degree. The castor oil modified polyester polyol is used for modifying polyurethane, so that the adhesive property and the heat resistance of the adhesive can be obviously improved. The adhesion problem of cracking at an adhesion position of wood during high-temperature baking processes such as wood drying and bent plate making in a wood processing process is solved.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI +1
Resin for Oxygen-absorbing Adhesive and Oxygen-absorbing Adhesive
InactiveUS20130143734A1Improve oxygen removal effectLow costNon-macromolecular adhesive additivesOther chemical processesPolyesterPolymer science
The purpose of the present invention is to provide a two-component curable oxygen-absorbing resin composition that has both oxygen-absorbing and adhesive properties and cohesive power. The resin for an oxygen-absorbing adhesive is a polyester comprising structural units derived from an acid component (A) and an acid component (B), wherein the ratio of the acid component (A) to total acid components is 70 to 95 mol %, the ratio of the acid component (B) to total acid components is 0 to 15 mol %, the polyester has a glass transition temperature of −20° C. to 2° C., the resin is cured using a hardening agent, the acid component (A) is tetrahydrophthalic acid or a derivative thereof, or tetrahydrophthalic acid anhydride or a derivative thereof, and the acid component (B) is phthalic acid.
Owner:TOYO SEIKAN GRP HLDG LTD
Method of making bisimides
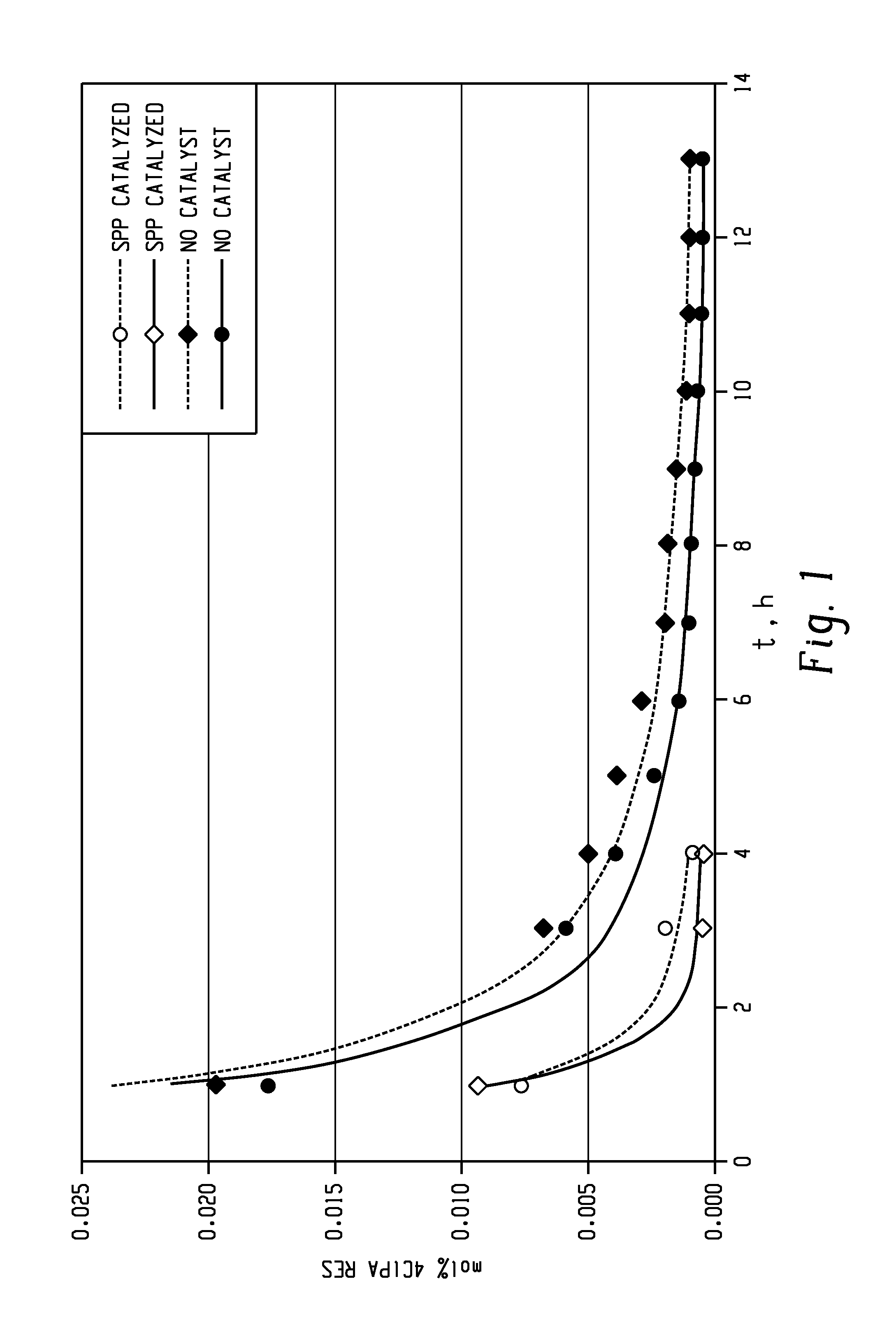
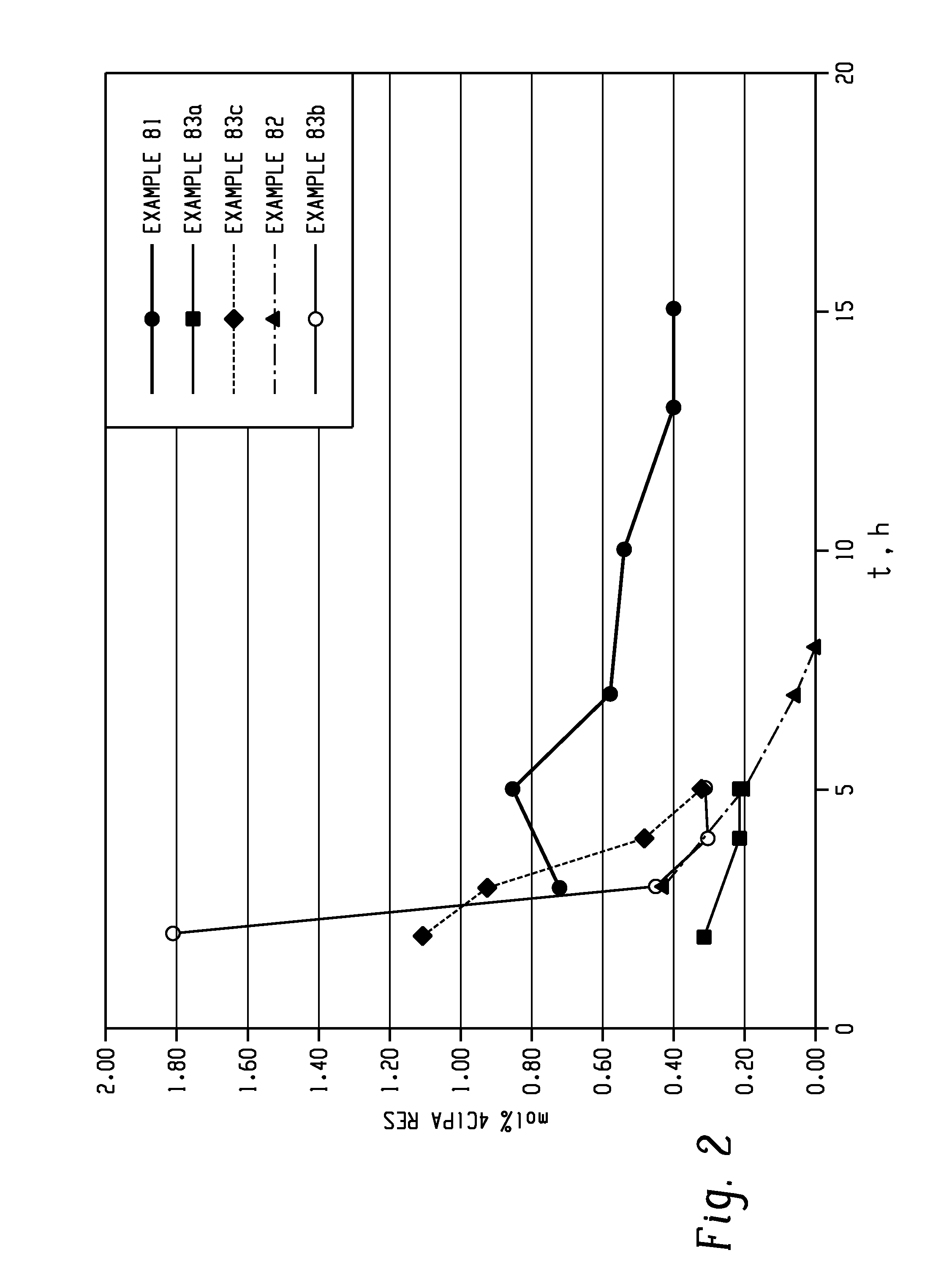
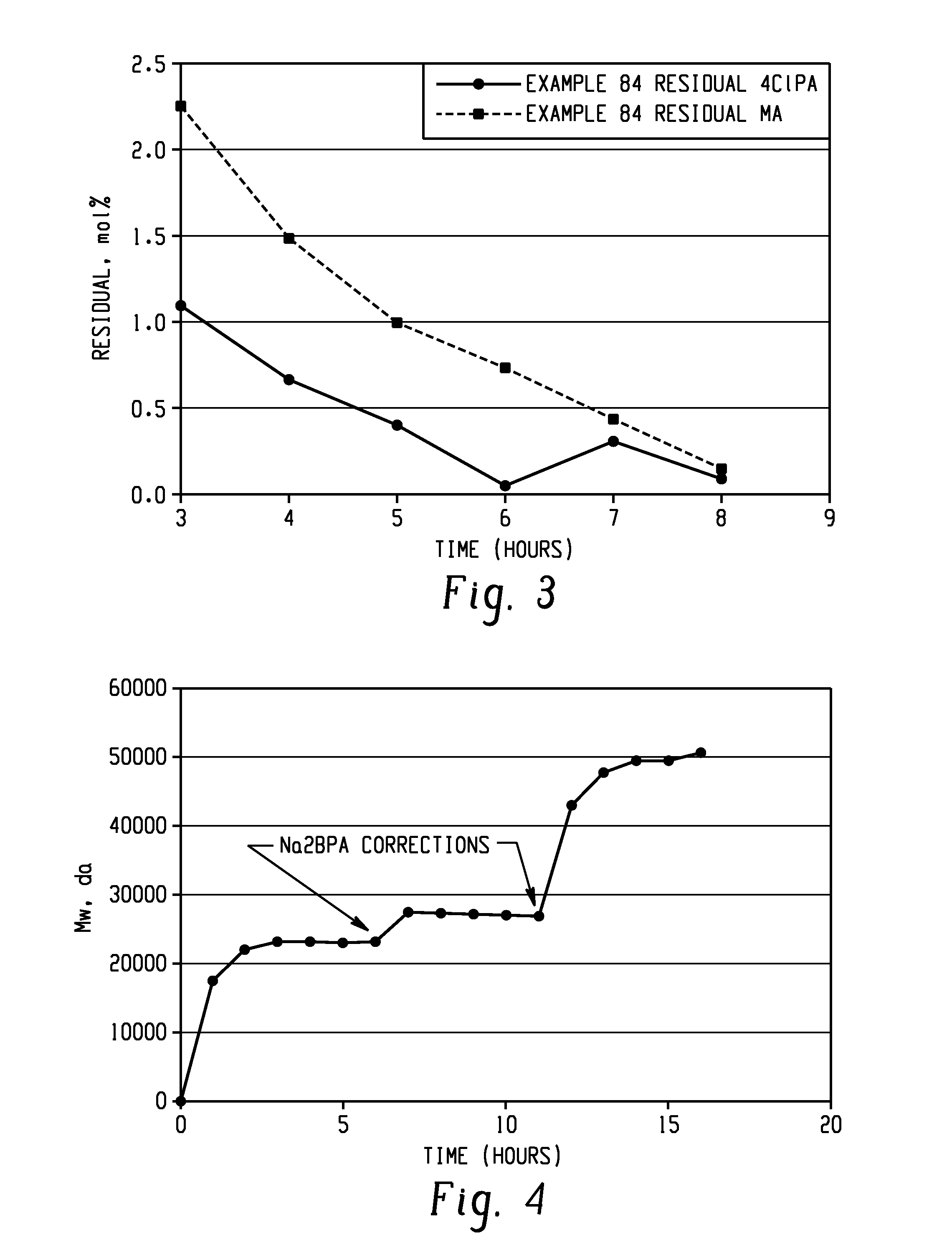
The present invention provides a method for preparing relatively insoluble bisimides under conditions which afford high imidization reaction rates and which permit the monitoring and adjustment of reactant stoichiometry at any stage of the reaction. The bisimides provided by the present invention are prepared either by reaction of a diamine such as 4,4′-diaminodiphenylsulfone (DDS) with an anhydride, for example 3-chlorophthalic anhydride (3-ClPA) in the presence of a solvent at a pressure greater than one atmosphere and at a temperature above the normal boiling point of the solvent, or by reaction of a monoamine with a dianhydride under the same conditions. In one embodiment, the relatively insoluble product bisimides provided by the present invention have a solubility in ortho-dichlorobenzene of less than about 10 percent by weight at a temperature of about 180° C.
Owner:SHPP GLOBAL TECH BV
Activator for chemical combination of phenol by benzene hydroxide radical and the application method
InactiveCN101032697AEasy to manufactureReuseOrganic chemistryOrganic compound preparationPtru catalystEthylic acid
The present invention discloses one kind of catalyst for hydroxylating benzene to synthesize phenol and its application method. The catalyst with molecular sieve as carrier is prepared through soaking the carrier to support salt of transition metal, roasting, mixing with phthalic anhydride, urea and ammonium molybdenate, and in-situ solid phase synthesis. It is used in the oxidation of benzene dissolved in solvent comprising acetic acid and water with oxygen as oxidant in the presence of reductant ascorbic acid to synthesize phenol. The catalyst of the present invention has no loss of active components, easy separation from the reaction system, mild reaction condition, high yield of target product phenol and other advantages.
Owner:EAST CHINA NORMAL UNIV
Hot melt adhesive composition
A hot melt adhesive composition comprising: (a) a functional copolymer obtained from copolymerization of ethylene and a comonomer selected from maleic anhydride, citraconic anhydride, itaconic anhydride, tetrahydrophthalic anhydride, maleic acid, citraconic acid, itaconic acid, fumaric acid, tetrahydrophthalic acid, the corresponding salts, monoesters and diesters of these acids, and mixtures of any of these, wherein the functional copolymer is present in an amount from about 5 to about 95 weight % of the combined total amount of (a) and (b); and (b) at least one ethylene copolymer obtained from copolymerization of ethylene with a polar monomer wherein said polar comonomer is present in the copolymer in an amount of from 8 to 40 weight %, wherein said ethylene copolymer is selected from the group consisting of ethylene / vinyl acetate copolymers, ethylene / alkyl (meth)acrylate copolymers and ethylene / alkyl (meth)acrylate / carbon monoxide terpolymers, in an amount from about 5 to about 95 weight % of the combined total amount of (a) and (b); wherein the composition has a melt index of 100 grams / 10 minutes or higher. Articles comprising and process using the hot melt adhesive are also disclosed.
Owner:DOW GLOBAL TECH LLC
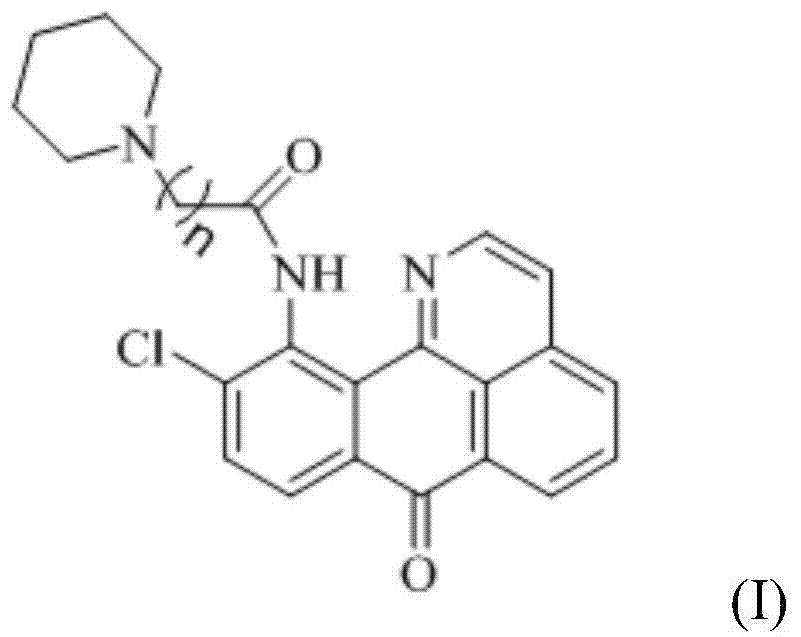
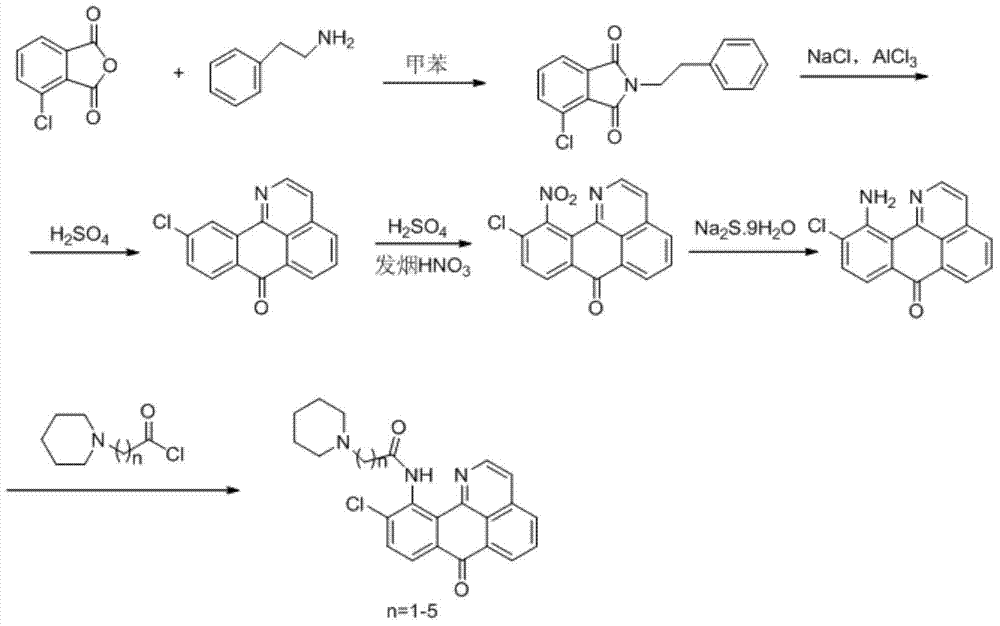
11-replaced oxoisoaporphine derivatives as well as synthetic method and application thereof
InactiveCN103923010AStrong inhibitory activityGood potential medicinal valueOrganic active ingredientsSenses disorderKetoneStructural formula
The invention discloses a series of 11-replaced oxoisoaporphine derivatives as well as a synthetic method and an application thereof. The synthetic method comprises the following steps: (1) carrying out ring closing reaction on 3-chlorophthalic anhydride and phenylethylamine as raw materials so as to construct a 10-Cl-1-azabenzanthrone parent body; (2) nitrating the parent body compound so as to obtain a 11-site nitrated product, and reducing the 11-site nitrated product so as to obtain 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone; and (3) reacting the 11-amino-10-chlorine-7H-dibenzoquinoline-7-ketone with an acyl chloride compound connected with piperidine so as to obtain a corresponding target product. Through study, the applicant finds that the series of derivatives have very strong inhibitory activity on acetylcholin esterase and are expected to be used for treating AD (Alzheimer Disease), cerebrovascular dementia and related diseases caused by cholinergic neurotransmitter reduction. The structural formula of the 11-replaced oxoisoaporphine derivatives is shown in descriptions.
Owner:GUANGXI NORMAL UNIV
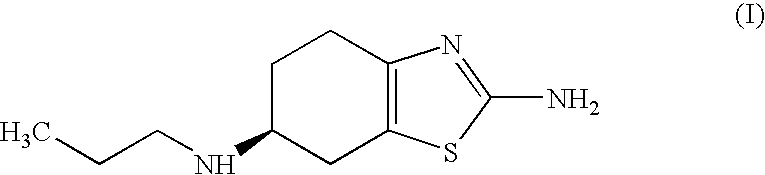
Process for the preparation of biologically active tetrahydrobenzthiazole derivative
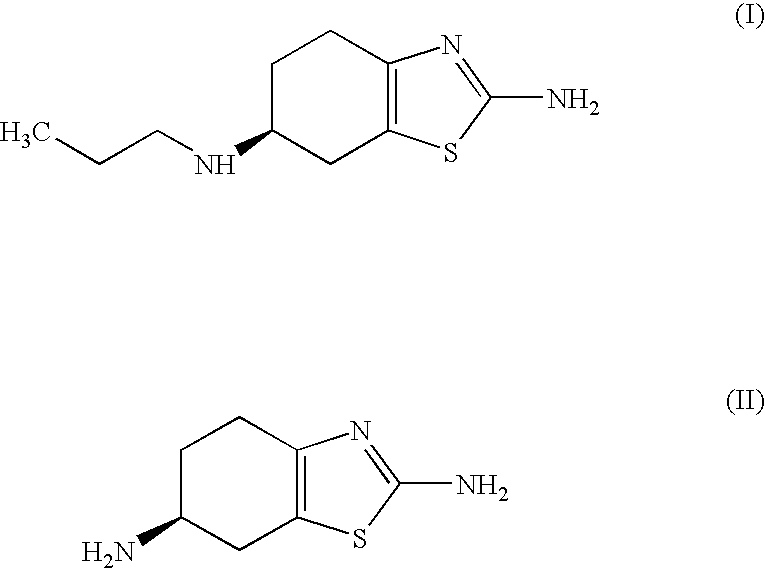
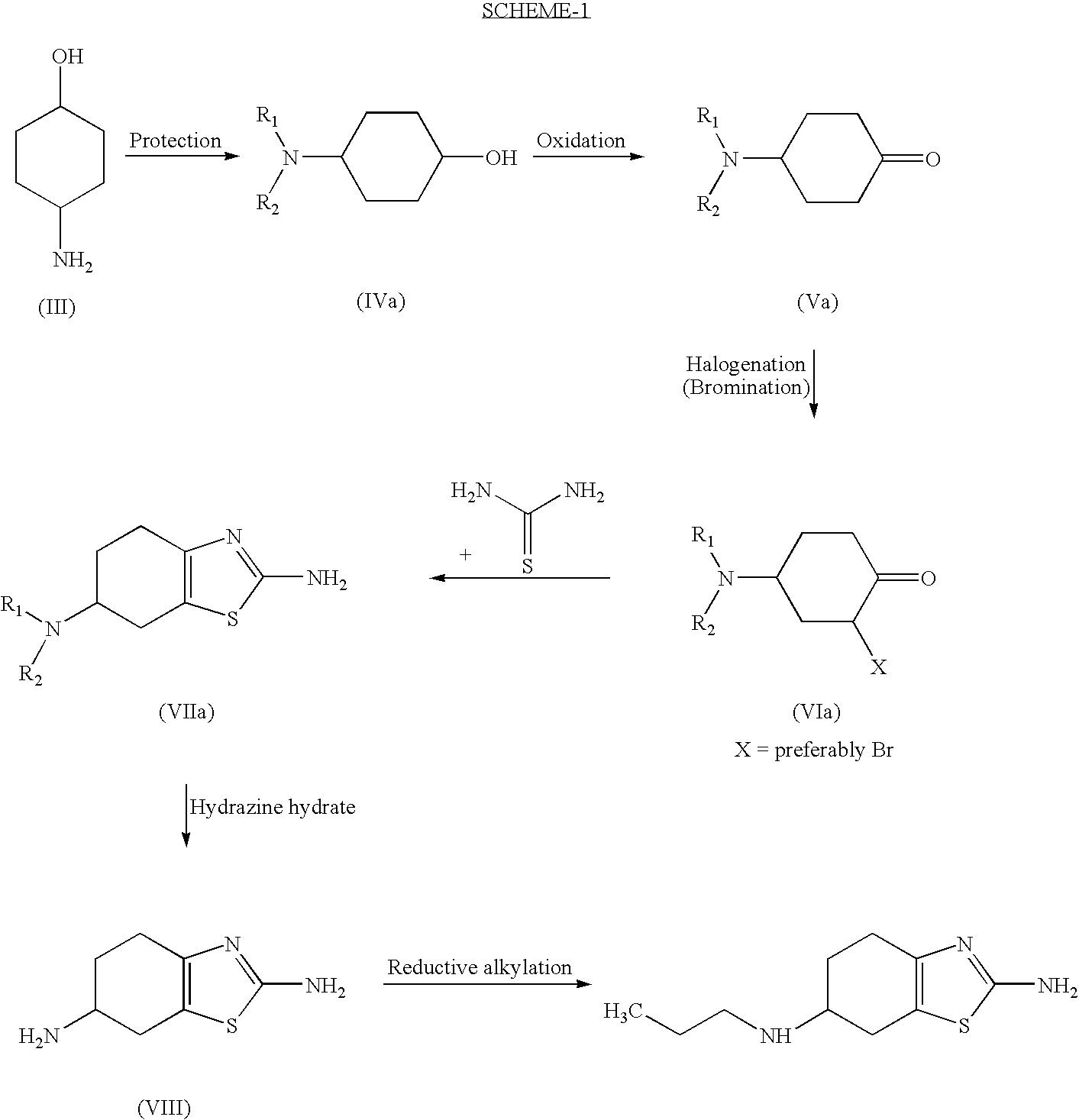
InactiveUS20070123573A1Shorten the setting timeOrganic active ingredientsBiocideCyclohexanoneThiourea
Improved process for the preparation of the intermediate compound of formula II for formation of biological active tetrahydrobenzothiazole compound of formula (I) as well as the biological active tetrahydrobenzothiazole compound of formula (I) and / or its pharmaceutically acceptable salts or solvates. The process comprises reacting 4-amino cyclohexanol of formula (III) or its acid addition salts with phthalic anhydride in presence of acid catalyst and their salts, in polar aprotic solvent or its mixture with organic solvent, capable of removing water azeotropically to give 4-(phthalimido)-cyclohexanol of formula (IV); oxidizing 4-(phthalimido)-cyclohexanol of formula (IV) to give 4-(phthalimido)-cyclohexanone of formula (V); brominating 4-(phthalimido)-cyclohexanone of formula (V) with brominating agent in organic solvent in presence of Lewis acid catalyst to prepare 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI); treating 2-bromo-4-(phthalimido)-cyclohexanone of formula (VI) with thiourea in organic solvent in presence of base to give 2-amino-6-phthalimido-4,5,6,7-tetrahydro benzothiazol of formula (VII); reacting compound of formula (VII) with hydrazine hydrate and base in polar solvent to give racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII); resolving racemic 2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (VIII) to prepare (6S)-2,6-diamino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II). To form the compound of Formula I and if desired its salts / solvates the above process is carried out with further steps of coupling (6S)-2,6-dimino-4,5,6,7-tetrahydro-1,3-benzothiazole of formula (II) with propionaldehyde in presence of mineral acid in polar organic solvent and reducing agent to prepare (S)-(−)-2-Amino-6-(n-propylamino)-4,5,6,7-tetrahydrobenzothiazole of formula (I);and if desired converting (S)-(−)-2-Amino-6-(propylamino)-4,5,6,7-tetrahydrobenzothiazole to its pharmaceutically acceptable salts or solvates.
Owner:ALEMBIC LTD
Antique paint and manufacturing technique thereof
The invention provides an antique paint and a manufacturing technique thereof, relating to the field of paints. The antique paint is composed of the following components in parts by weight: 5-25 parts of epoxy resin, 2-6 parts of phthalic anhydride, 10-15 parts of polyacrylamide, 8-20 parts of isopropanol, 4-10 parts of titanium white, 2-5 parts of nano silver oxide, 0.1-0.4 part of sodium soap, 5-8 parts of light calcium carbonate powder, 3-5 parts of kaolin, 0.7-2 parts of coupling agent, 0.5-6 parts of dispersing agent, 4-8 parts of defoaming agent, 3-10 parts of anti-settling agent, 3-8 parts of thickener, 1-3 parts of pigment, 5-25 parts of thinner, 5-15 parts of metal powder, 5-8 parts of alkyd resin and 55-75 parts of turpentine. The coated antique paint provided by the invention has the advantages of low cracking tendency, favorable glossiness and favorable decorative effect.
Owner:DONGGUAN LIUHUA ART
Poly(imide-amide) copolymer, article containing poly(imide-amide) copolymer, and electronic device including same
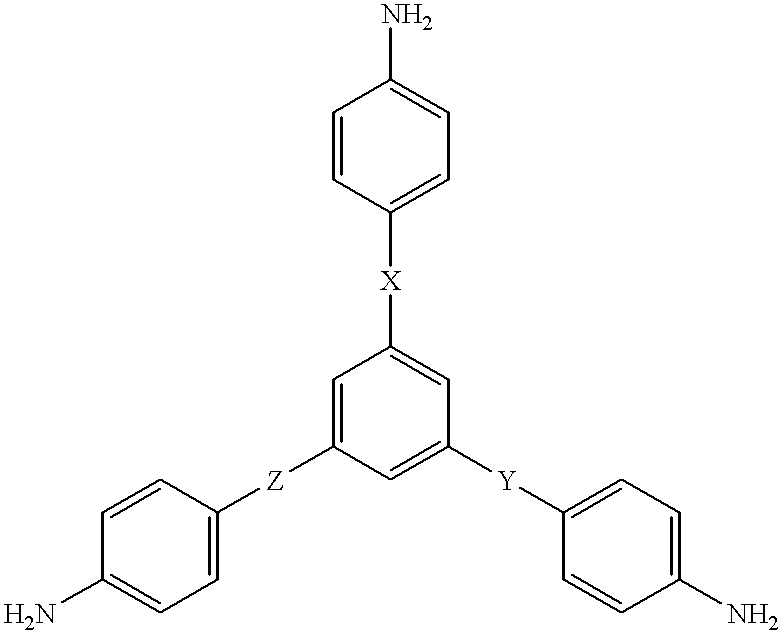
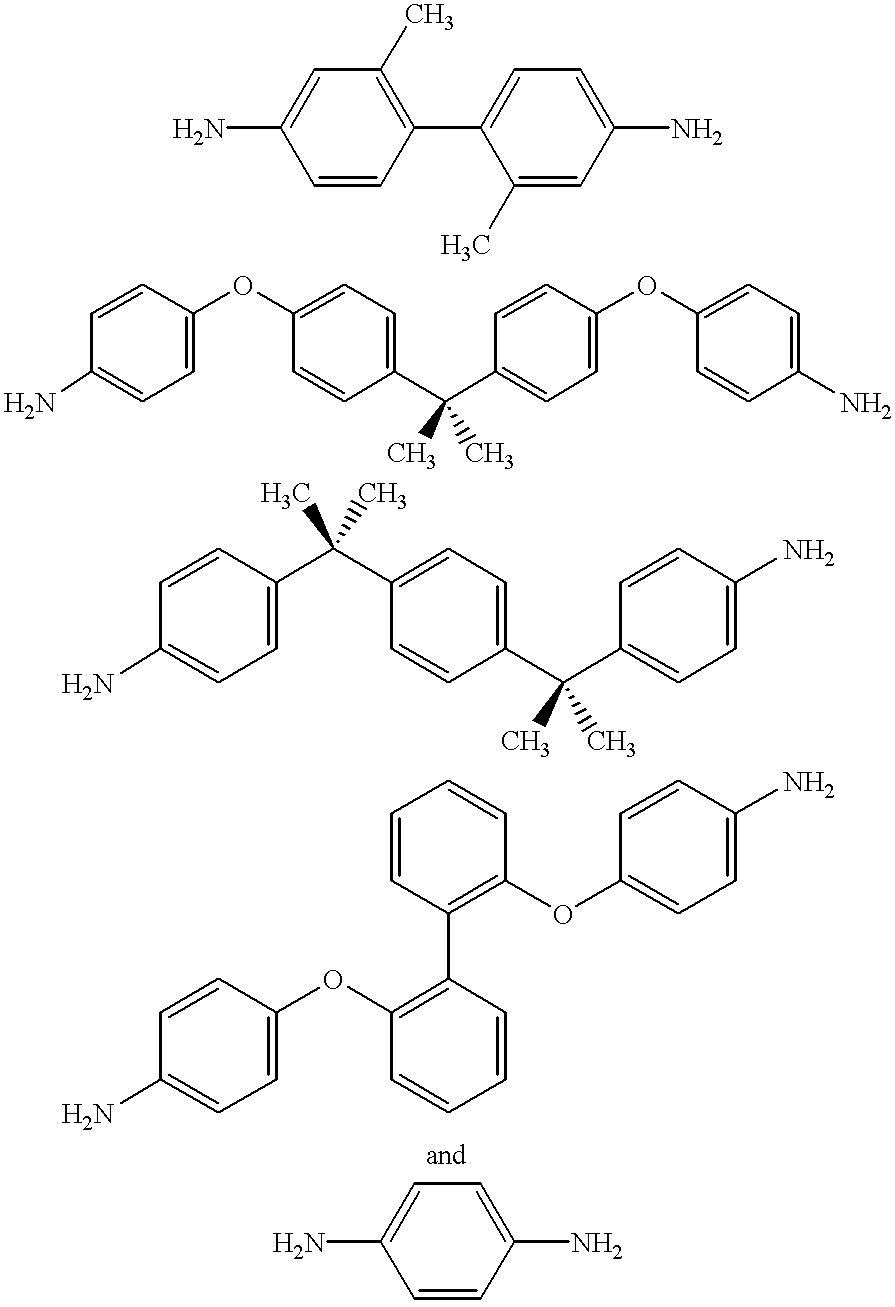
A poly(imide-amide) copolymer includes: an imide structural unit which is a reaction product of a first diamine and a dianhydride, and an amide structural unit which is a reaction product of a second diamine and a diacyl halide, wherein each of the first diamine and the second diamine includes 2,2'-bis-trifluoromethyl-4,4'-biphenyldiamine, and at least one of the first diamine and the second diamine further includes a compound represented by Chemical Formula 1, wherein the dianhydride includes 3,3',4,4-biphenyltetracarboxylic dianhydride and 4,4'-hexafluoroisopropylidene diphthalic anhydride, wherein the diacyl halide includes terephthaloyl chloride (TPCI), and wherein an amount of the compound represented by Chemical Formula 1 is less than or equal to about 10 mole percent based on the total amount of the first diamine and the second diamine: NH2-A-NH2 Chemical Formula 1, wherein in Chemical Formula 1, A is the same as described in the detailed description.
Owner:SAMSUNG ELECTRONICS CO LTD +1
Polyamic acid solution composition and polyimide film
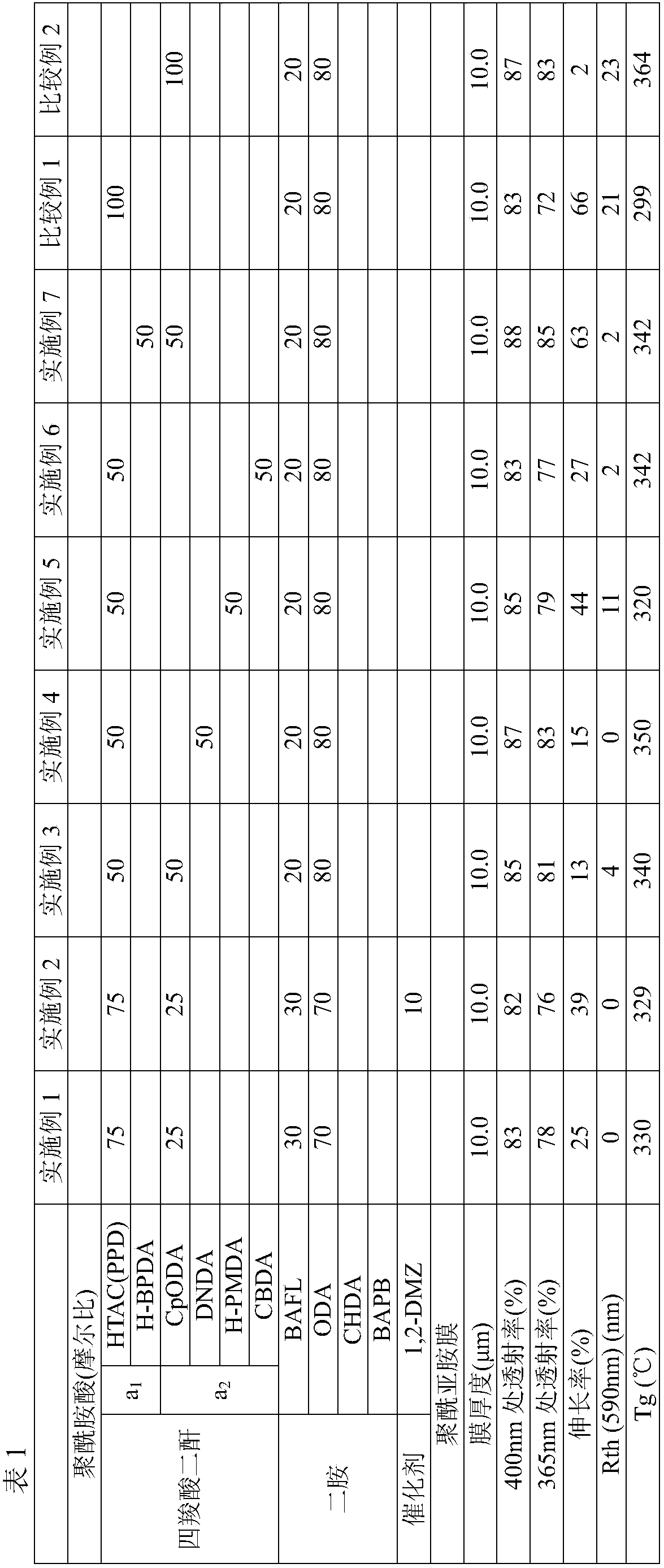
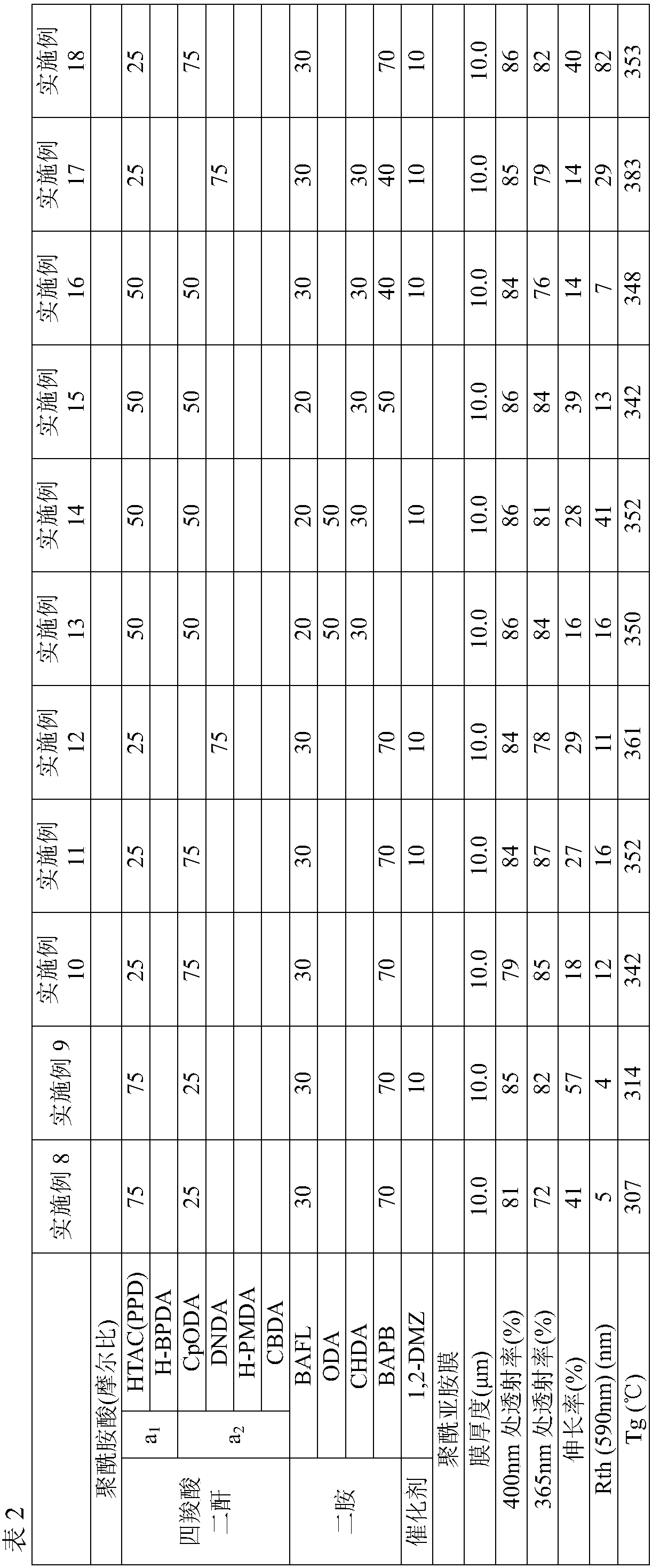
ActiveCN107949597AGood light transmissionSmall phase differenceCoatingsCircuit susbtrate materialsImidePolymer science
The invention discloses a polyamic solution composition and a polyimide film. The present invention pertains to a polyimide film comprising mainly a polyimide obtained by polymerizing a tetracarboxylic acid component and a diamine component, wherein the tetracarboxylic acid component consists of one or more phthalic anhydride structure-free tetracarboxylic dianhydrides (a1) in which at least one of the bonds connecting the two cyclic acid anhydride structures in the molecule is a freely rotatable bond, and one or more tetracarboxylic dianhydrides (a2) with no freely rotatable bonds in the molecule having an alicyclic structure and the two cyclic acid anhydride structures sharing at least one carbon-carbon bond with an alicyclic structure, and the diamine component includes 5-50 mol% of oneor more diamines having a 9,9-diphenylfluorene structure.
Owner:UBE IND LTD
Natural plant type paint and its preparation method
The present invention relates to a natural plant type coating and its preparation method. Said coating is formed from high-molecular film-forming material, pigment, adjuvant and solvent. It is characterized by that the oil material adopted in the high-molecular film-forming material contains 30-40 portions of linseed oil, 35-45 portions of limonene, 5-15 portions of polybasic alcohol, the polybasic acid contains 8-15 portions of phthalic anhydride and 2-5 portions of trimellitic anhydride, the adjuvant is 0.02-0.1 portion of catalyst lithium hydroxide, the dryer is 1-3 portions of composite rare earth, and the solvent contains 5-10 portions of pine cone oil and 5-10 portions of terpineol, the above-mentioned raw materials are mixed to obtain the invented film-forming material. Besides, it adopts deionized water, adds 18-35 portions of pigment titanium dioxide, adds drying, dispersing, anti-freezing, defoaming, anti-corrosion and film-forming adjuvants, and adds medicinal talcum powder and natural calcium carbonate and stirs them to obtain slurry material for stand-by, then said invention utilizes 10-20 portions of film-forming material to make emulsification, adds deionized water and the above-mentioned slurry material, and adds thickening agent, mixes them so s to obtain the invented coating.
Owner:TIANJIN LIUHONG TECH DEV
Analysis method for polyether polyol hydroxyl value
InactiveCN103048322ASimplify analysis stepsHigh precisionMaterial analysis by observing effect on chemical indicatorTest samplePotassium hydroxide
The invention provides an analysis method for polyether polyol hydroxyl value, which comprises the following steps: A, weighing a polyether polyol test sample m into a conical flask, and taking another empty conical flask; B, transferring a phthalic anhydride pyridine solution into the test sample accommodated conical flask and the empty conical flask respectively, placing the two conical flasks in an oscillation oil bath pan, and connecting the two conical flasks with an air condensation pipe for reaction; C, after reaction, taking the two conical flasks out of the oil bath pan for cooling; D, flushing the two conical flasks with deionized water and acetone in sequence, and oscillating the two conical flasks to mix the solutions completely; E, adding a sodium hydroxide aqueous solution into the two conical flasks respectively, and adding a few drops of phenolphthalein indicator; F, titrating the solutions in the two conical flasks with potassium hydroxide standard solution until the solutions in the two conical flasks changes into pink; and G, calculating out the polyether polyol hydroxyl value as per a formula. The method is simple in analysis procedure, has high precision and accuracy, can efficiently shorten detection period, lower experimental cost, and bring certain economical benefits to an enterprise.
Owner:SHANGHAI FALAB TEST
Low viscosity polyester-polyols and polyurethane foams prepared therefrom
InactiveUS6284811B1High tensile strengthImprove securityIsocyanic acid derivatives preparationCarbamic acid derivatives preparationPolyesterPolymer science
A polyester-polyol being liquid at 40° C. and having a viscosity of not more than 10,000 mPa.s at 60° C., prepared by polycondensation of an acid component including at least one phthalic acid component selected from phthalic anhydride and o-phthalic acid, and an aliphatic polybasic acid, with a polyhydric alcohol; a process for preparing a polyurethane foam including reacting a polyisocyanate component with a polyol component including the polyester-polyol; a process for preparing an isocyanate prepolymer from a polyisocyanate component and a polyol component including the polyester-polyol; and a process for preparing a polyurethane foam including reacting an isocyanate prepolymer prepared from a polyisocyanate component and a polyol component including the polyester-polyol, with a polyol component. The resulting polyurethane foam shows remarkably improved physical properties such as tensile strength and tear strength, while having low density, acceptable appearance and texture.
Owner:KAO CORP
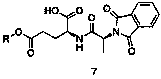
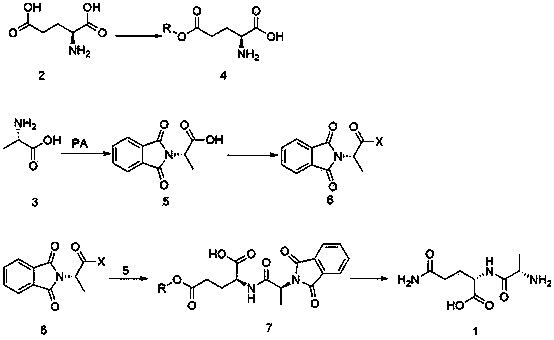
New preparation method of L-alanyl-L-glutamine
The invention provides a new preparation method of L-alanyl-L-glutamine. The new preparation method comprises the following steps of: reacting phthalic anhydride with L-alanyl so as to prepare phthalic anhydride-L-alanyl, and acylating and halogenating the phthalic anhydride-L-alanyl with a halogenated reagent so as to synthesize phthalic anhydride-L-alanyl halogen; carrying out esterification reaction on L-glutamic acid and alcohol under the action of acid catalysis to obtain L-glutamic acid monoester; condensing the L-glutamic acid monoester and the phthalic anhydride-L-alanyl halogen under the action of alkali to obtain phthalic anhydride-L-alanyl-L-glutamic acid monoester which is an important intermediate for synthesizing the L-alanyl-L-glutamine; adding the phthalic anhydride-L-alanyl-L-glutamic acid monoester to ammonium hydroxide for ammonolysis and deprotection, and finally crystallizing in alcohol to obtain L-alanyl-L-glutamine. The new preparation method has the advantages that reaction raw materials are easily available, L-glutamic acid and L-alanyl are low in price, the product purity and the product yield are high, the production cost can be effectively reduced, and large-scale production is realized.
Owner:CONSCI PHARMA
A fast-drying alkyd resin used for wood lacquers, and a preparation method thereof
InactiveCN103232593AHigh molecular weightLess free hydroxylPolyester coatingsBenzoic acidPolymer science
The invention belongs to the technical field of coating resin, and in particular relates to a fast-drying alkyd resin used for wood lacquers, and a preparation method thereof. The fast-drying alkyd resin used for wood lacquers comprises the following components, by weight: 17-21 parts of vegetable oil acid, l 4-9 parts of ethylene glycol, 9-18 parts of pentaerythritol, 21-30 parts of phthalic anhydride, 0.6-1.3 parts of maleic anhydride, 2-7 parts of benzoic acid, 3-9 parts of a chain extender, 0.04-0.09 parts of an antioxidative color-reducing agent, and 23-39 parts of a letdown solvent. According to the invention, the alkyd resin has a large molecular weight, relatively few free hydroxyl groups, and an overall uniform molecular weight distribution, so the fast-drying alkyd resin used for wood lacquers of the present invention has characteristics of fast drying, good transparency, good polishing property, and a high performance cost ratio.
Owner:SKSHU PAINT
Method for preparing chitosan oligosaccharide modified biodegradable composite
ActiveCN103254599AReduce crystallinityRing-opening polymerization is easy to realizePolymer scienceRing-opening polymerization
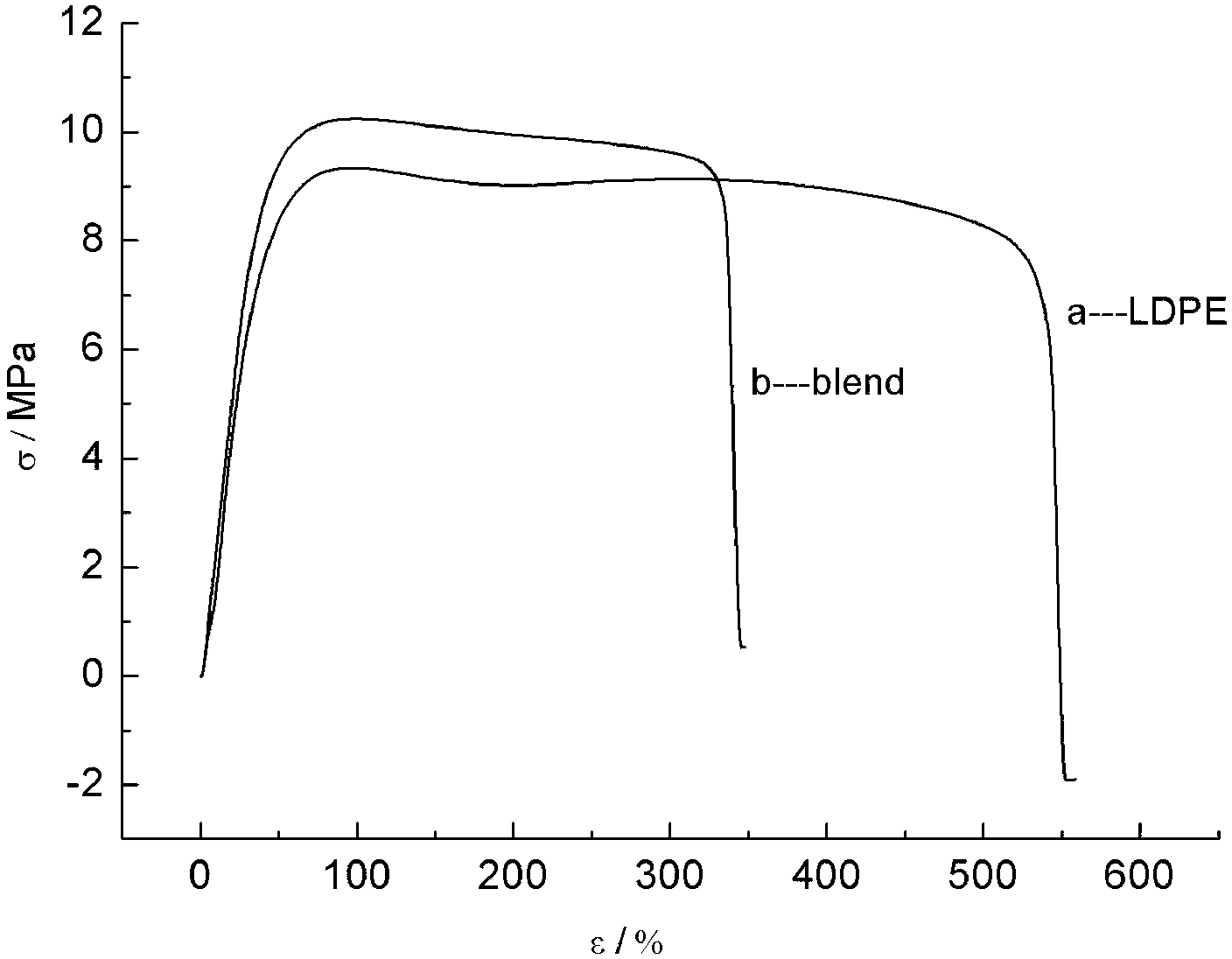
The invention mainly relates to a method for preparing a chitosan oligosaccharide modified biodegradable composite. The method comprises the steps of: firstly, with N,N-dimethyl formamide as a solvent, protecting amino of chitosan oligosaccharide by using phthalic anhydride to prepare a N-methyl o-acyl chitosan oligosaccharide reaction intermediate; secondly, dropwise adding pyridine solution of stannous octoate under protection of nitrogen, initiating an epsilon-caprolactone monomer to carry out ring opening polymerization so that polycaprolactone is grafted on hydroxyl of N-methyl o-acyl chitosan oligosaccharide, so as to generate a chitooligo saccharide-grafted polycaprolactone thermoplastic material; and finally melting and blending LDPE (low-density polyethylene), chitooligo saccharide-grafted caprolactone and polycaprolactone to prepare the biodegradable composite. The blended composite has biological degradability, and is free of poison and wide in application range; deterioration of an ecological environment is effectively improved; and the situations of energy crisis and petroleum resource shortage are solved.
Owner:SHENYANG POLYTECHNIC UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com