Catalyst for propylene polymerization and its preparation method
A technology of propylene polymerization and catalyst, which is applied in the field of catalyst components and its preparation, can solve the problem of few catalyst patents, and achieve the effect of simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The preparation of catalyst involved in the present invention comprises following two parts:
[0015] Preparation of A complex:
[0016] In the glass reaction flask fully replaced by nitrogen, add 5g of anhydrous MgCl 2 And 40ml200# solvent naphtha and 24ml isooctyl alcohol. The stirring was started and the temperature was raised, and the temperature was maintained at 130° C. for 2 hours. Add 0.75ml of tetrabutyl titanate and 1g of phthalic anhydride into the reaction flask, react at this temperature for 2 hours to obtain a homogeneous complex solution, and cool to room temperature.
[0017] Titanation of B complex:
[0018] The homogeneous solution prepared by the above method was dropped into a glass reactor containing a mixture of 200ml Ticl4 and 10ml refined toluene at -20°C within 30 minutes, and kept under stirring for 0.5h. After 4 hours, the temperature was raised to 108°C. At this temperature, 1.5ml of diisobutyl phthalate was added, and the temperature was...
Embodiment 2
[0027] The preparation of A complex is the same as in Example 1.
[0028] Titanation of B complex:
[0029] The homogeneous solution prepared by the above method was dropped into a glass reactor containing 200ml of Ticl4 and 20ml of refined 200# oil mixture at -20°C within 30 minutes, and kept under stirring for 0.5h. After 4 hours, the temperature was raised to 108°C. At this temperature, 1.5ml of diisobutyl phthalate was added, and the temperature was continued to rise to 110°C for 2 hours. After the liquid was filtered out, 200ml of Ticl4 was added again, and the temperature was raised to 110°C for 2 hours. After the reaction, the liquid was filtered off, washed once with refined toluene at 80°C, and then washed with hexane less than 2PPm until there were no free chloride ions in the filtrate. The remaining solid product was vacuum dried to obtain a solid catalyst component.
[0030] Polymerization test is the same as example 1.
Embodiment 3
[0032] The preparation of A complex is the same as in Example 1
[0033] Titanation of B complex:
[0034] The homogeneous solution prepared by the above method was dropped into a glass reactor containing 1.2ml of Ticl4 and 80ml of refined 200# oil mixture at -20°C within 30 minutes, and kept under stirring for 0.5h. Increase the stirring speed to 800 rpm, heat up to 108°C after 4 hours, add 1.5ml of diisobutyl phthalate at this temperature, continue to heat up to 110°C, react for 2 hours, filter out the liquid, and then add 200ml Ticl4 was heated again to 110°C, and reacted for 2 hours. After the reaction, the liquid was filtered off, washed once with refined toluene at 80°C, and then washed with less than 2PPm hexane until there were no free chloride ions in the filtrate. The remaining solid product was vacuum dried to obtain a solid catalyst component.
[0035]
[0036]
[0037]
[0038]
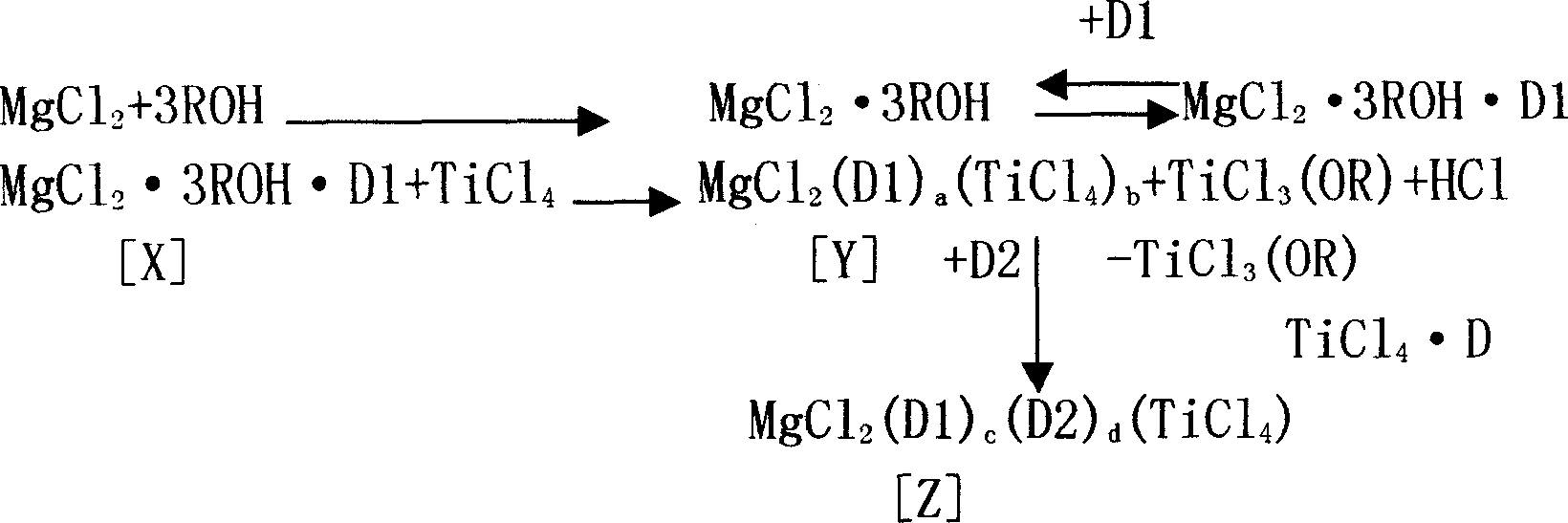
[0039] D1 and D2 represent esters, and ROH represents alcoh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com