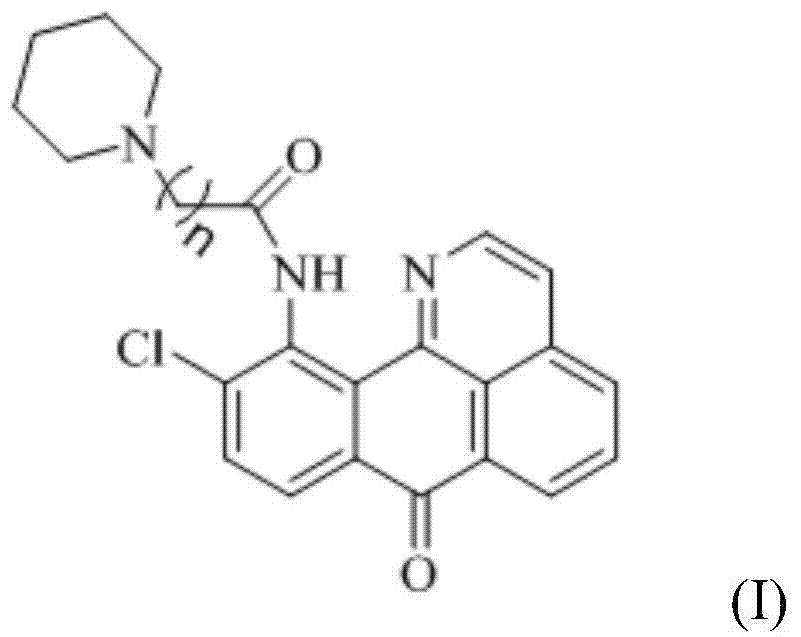
11-replaced oxoisoaporphine derivatives as well as synthetic method and application thereof
A technology of isoaporphine and a synthesis method, which is applied in the field of 11-substituted oxidized isoapophthene derivatives and the synthesis fields thereof, can solve the problems that have not been seen before, and achieve the effects of good medicinal value and strong inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
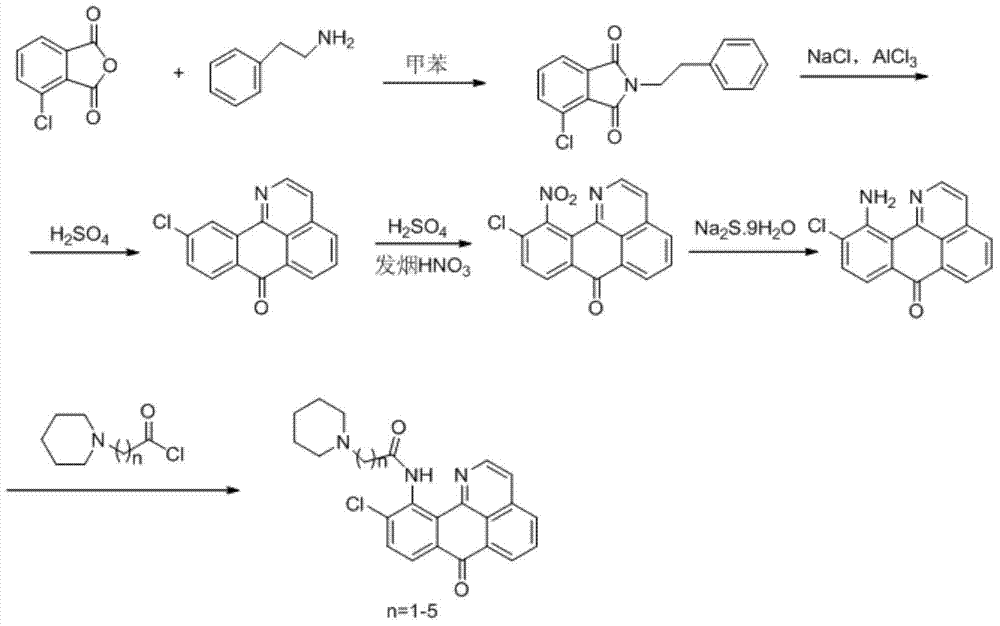
[0036] Example 1: Synthesis of 4-chloro-2-phenylisoindoline-1,3-dione (compound 1)
[0037] Add 57g (0.1mol) of 3-chlorophthalic anhydride and 45g (0.1mol) of phenethylamine into a 1L three-hole round bottom flask, then add 500ml of toluene, reflux for 6 hours, pour it out while it is hot, and cool it down. Crystals were precipitated and filtered by suction to obtain compound 1 (white flaky crystals) with a yield of about 98%.
[0038] Compound 1 is analyzed, and its spectral characteristics are as follows:
[0039] 1 H NMR (CDCl 3 ,500MHz): δ3.00~3.04(m,2H),3.94~3.97(m,2H),7.23~7.34(m,5H),7.65(d,J=1.7,1H),7.66(s,1H) ,7.78(dd,J 1 =5.0andJ 2 =3.2,1H);ESI-MS(m / z):287[M+H] + .
[0040] Therefore, it can be determined that the above-mentioned compound 1 is 4-chloro-2-phenylisoindoline-1,3-dione, and its structural formula is as follows:
[0041]
Embodiment 2
[0042] Embodiment 2: Synthesis of 10-Cl-1-azabenzanthrone (compound 2)
[0043] Add 75g (0.56mol) of anhydrous aluminum trichloride and 15g of sodium chloride into a 1L three-hole round-bottomed flask, mix and heat to 140°C for melting, then slowly add 53g (0.2mol) of compound 1 in batches After the addition, the temperature was raised to 220°C, and the reaction was stirred for 3 hours, poured into a mortar while heating, cooled, and crushed to obtain a reddish-brown solid, which was placed in a dry vessel.
[0044] Take 600ml of concentrated sulfuric acid and add it to a 2L three-hole round bottom flask. When the temperature rises to 80°C, start adding the reddish-brown solid obtained in the previous step in batches. into about 600g of ice water, adjust the pH of the system to about 2-3 with NaOH, a large amount of solids precipitated, filtered with suction, and washed with water to obtain a crude product. The crude product was purified by silica gel column chromatography (p...
Embodiment 3
[0049] Example 3: Synthesis of 11-amino-10-chloro-7H-dibenzoquinolin-7-one (compound 3)
[0050] Take 6mL of concentrated sulfuric acid, slowly add 4g of compound 2, after it is completely dissolved, then add 3mL of fuming nitric acid, stir at room temperature for 20min, then react at 50°C for 4h, stop the reaction. After cooling, pour it into about 150mL of ice water, filter it with suction, and dry it. A yellow 11-position nitrated product was obtained with a yield of about 85%.
[0051] Take the nitration product obtained in the previous step, 6.7g of sodium sulfide nonahydrate and 2.6g of NaOH in a 250mL round bottom flask, then add 200mL of ethanol solution (ethanol: water (volume ratio) = 7:3), stir and reflux for 4.5h. After stopping the reaction, cool down, distill ethanol off under reduced pressure, wash with water, and filter with suction to obtain compound 3 (dark red solid), with a yield of about 35%.
[0052] Compound 3 was analyzed, and its spectral characteris...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com