A kind of preparation method of halogenated alkanes synthesized with styrene and its derivatives and trichloroalkane
A technology of trichloroalkanes and halogenated alkanes, applied in the field of organic chemical synthesis, can solve the problems of low content, unfavorable drug discovery, difficult extraction and research, etc., to reduce emissions, ensure the health of operators, and make strong substrates universal sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
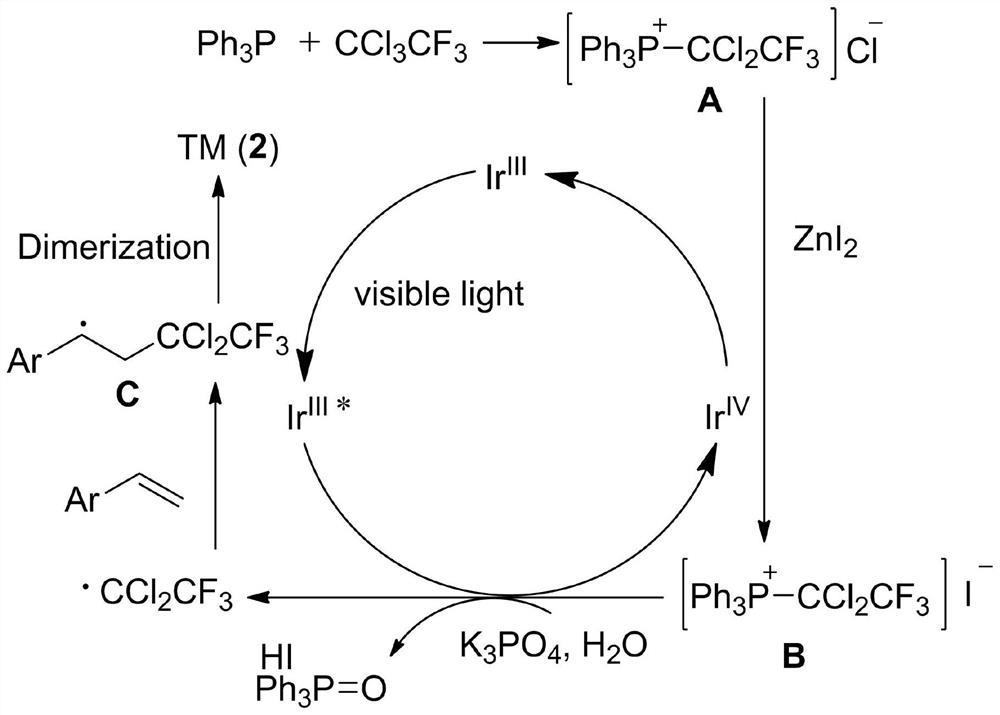
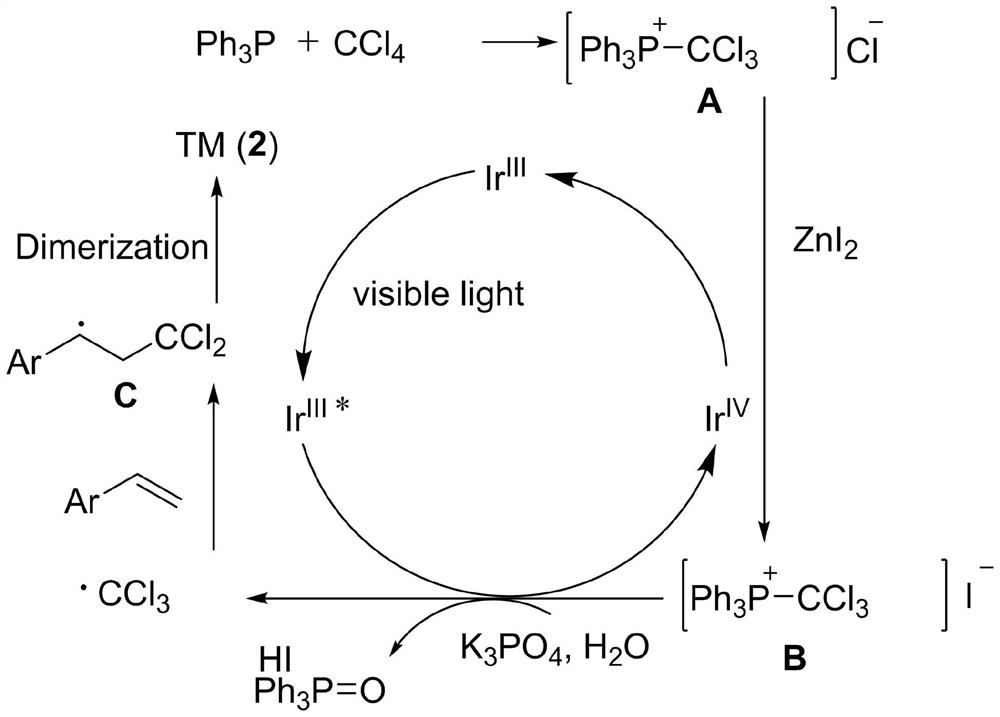
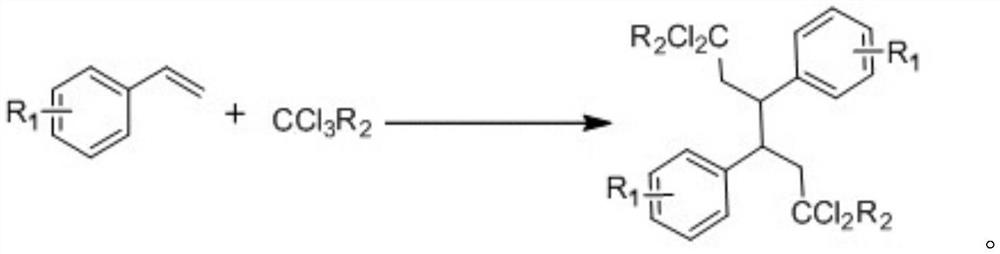
[0029] Specific embodiment one: under visible light irradiation, the styrene of 0.2mmol, the trifluorotrichloroethane of 1.0mmol, the light redox catalyst fac-[Ir(ppy) of 0.006mmol 3 ], 2.0mmol of deionized water, 2.0mmol of triphenylphosphine, 1.0mmol of zinc iodide, and 1.0mmol of potassium phosphate were added to 2.0ml of tetrahydrofuran for reaction. Stir for 12 hours under a nitrogen atmosphere at a temperature of 25°C. After the reaction was over, hydrogen peroxide was added dropwise into the flask until the solution turned purple. Then, ethyl acetate was added to the mixture, washed with saturated sodium thiosulfate and brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was purified by flash column chromatography to give 32.8 mg of white solid 2,2,7,7-tetrachloro-1,1,1,8,8,8-hexafluoro-4,5-diphenyloctane, Yield 64%. m.p.122-124℃. 1 H NMR (500MHz,...
specific Embodiment 2
[0030] Specific embodiment two: under visible light irradiation, the 4-methylstyrene of 0.2mmol, the trifluorotrichloroethane of 1.0mmol, the photoredox catalyst fac-[Ir(ppy) of 0.006mmol 3 ], 2.0mmol of deionized water, 2.0mmol of triphenylphosphine, 1.0mmol of zinc iodide, and 1.0mmol of potassium phosphate were added to 2.0ml of tetrahydrofuran for reaction. Stir for 12 hours under a nitrogen atmosphere at a temperature of 25°C. After the reaction was over, hydrogen peroxide was added dropwise into the flask until the solution turned purple. Then, ethyl acetate was added to the mixture, washed with saturated sodium thiosulfate and brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was purified by flash column chromatography to give 29.7 mg of white solid 2,2,7,7-tetrachloro-1,1,1,8,8,8-hexafluoro-4,5-bis(4-methyl Phenyl)octane, 55% yield. m.p.84-86℃(u...
specific Embodiment 3
[0031] Specific embodiment three: under visible light irradiation, the 3-methylstyrene of 0.2mmol, the trifluorotrichloroethane of 1.0mmol, the photoredox catalyst fac-[Ir(ppy) of 0.006mmol 3 ], 2.0mmol of deionized water, 2.0mmol of triphenylphosphine, 1.0mmol of zinc iodide, and 1.0mmol of potassium phosphate were added to 2.0ml of tetrahydrofuran for reaction. Stir for 12 hours under a nitrogen atmosphere at a temperature of 25°C. After the reaction was over, hydrogen peroxide was added dropwise into the flask until the solution turned purple. Then, ethyl acetate was added to the mixture, washed with saturated sodium thiosulfate and brine. After extracting the aqueous layer with ethyl acetate, the combined organic layers were dried over anhydrous sodium sulfate and evaporated under vacuum. The residue was purified by flash column chromatography to give 36.2 mg of white solid 2,2,7,7-tetrachloro1,1,1,8,8,8 hexafluoro-4,5-bis(3-methylphenyl ) octane, yield 67%. m.p.75-77℃...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com