Organic light-emitting nitrogen free radical molecule based on nitrogen heterocyclic structure and preparation method of organic light-emitting nitrogen free radical molecule
A nitrogen heterocycle and free radical technology, applied in the field of organic light-emitting nitrogen free radical molecules based on nitrogen heterocycle structure and its preparation, can solve the problems of restricting research and expanding applications in the field of organic light-emitting free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides the preparation method of the organic light-emitting nitrogen radical molecule based on the nitrogen heterocyclic structure described in the above technical scheme, comprising the following steps:
[0053] Dissolve the phenylhydrazine compound shown in formula A and the compound shown in formula B, and carry out an aldehyde-aromatic hydrazine condensation reaction to obtain a hydrazone compound;
[0054] Mix the aniline compound shown in formula C, water, concentrated hydrochloric acid and sodium nitrite aqueous solution, carry out diazotization reaction, obtain diazonium salt solution;
[0055] Mixing the hydrazone compound, inorganic base, phase transfer catalyst, organic solvent and the diazonium salt solution, and carrying out a diazonium salt coupling reaction to obtain the formazan compound;
[0056] The formazan compound, barium hydroxide and benzyl bromide are mixed, and a ring closure reaction and an oxidation reaction are ca...
Embodiment 1
[0091] Example 1: Synthesis of Compound 1
[0092] first step
[0093]
[0094] Add 1.06g (5mmol) of 2,4,6-trichlorophenylhydrazine to a 100mL single-necked flask, add 20mL of methanol under stirring to form a suspension, and dropwise add 0.56mL (10mmol) of acetaldehyde (the drop rate is 15mL / min) , gradually became clear, after 2 hours of reaction, the solvent was spin-dried to obtain yellow-brown colloidal solid product 1-1, the yield was 100%.
[0095] second step
[0096]
[0097] 1.1 g (5 mmol) of p-iodoaniline, 0.93 g (5.5 mmol) of carbazole, 1.2 g (15 mmol) of nano-copper oxide were added to a 250 mL one-necked flask, and 50 mL of DMF was added. The reaction was heated at 160 °C for 36 h with stirring. After cooling, suction filtration, pour the filtrate into 500 mL of water, and add 10 g of solid sodium chloride. Stir and dissolve, let stand for 30 min, and filter with suction. Add THF to the filter cake to dissolve and spin dry. Using petroleum ether:ethyl...
Embodiment 2
[0110] Example 2: Synthesis of Compound 2
[0111]
[0112] The synthesis steps are the same as the synthesis of compound 1, except that the raw material 2,4,6-trichlorophenylhydrazine in the first step reaction is replaced with phenylhydrazine to synthesize the intermediate product 2-1, GC-MS (m / z): [M]calculated for C 8 H 10 N 2 , 134.18, found, 134.14.
[0113] The subsequent steps are the same as the synthesis of compound 1, and the product 2 is finally obtained.
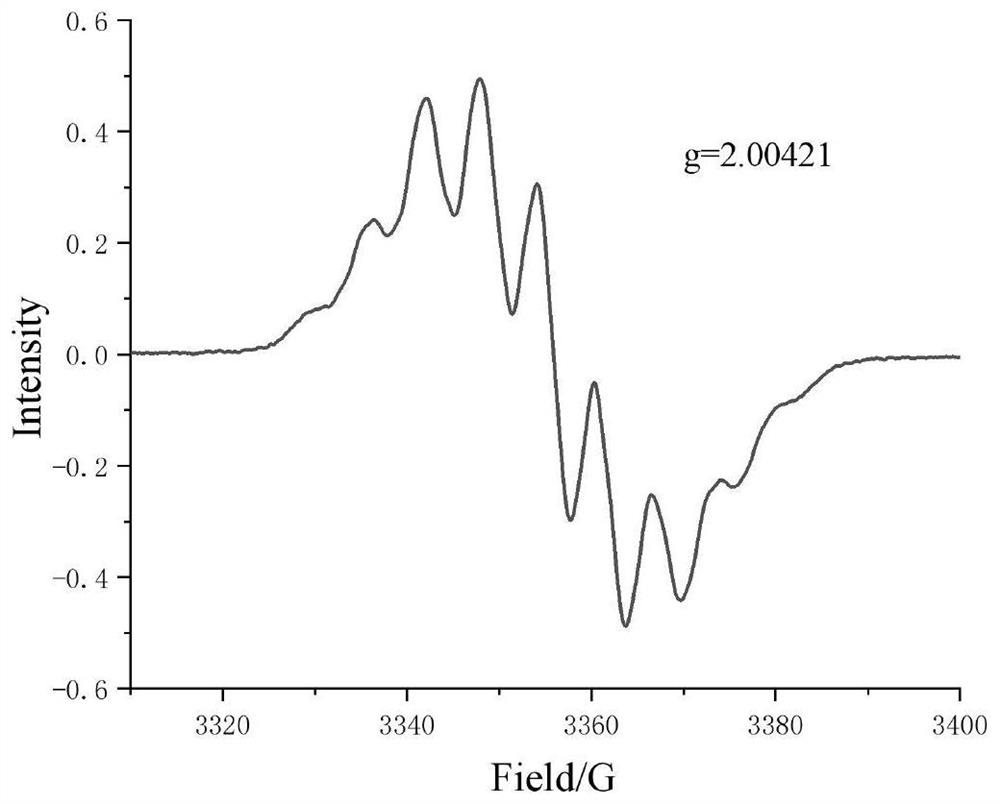
[0114] Image 6 is the EPR pattern of the obtained compound 2, from Image 6 It can be seen that: peak shape, hyperfine coupling splitting characteristics and g factor value (g=2.00391) are highly similar to compound 1, and are also consistent with the EPR signal characteristics of Verdazyl radical, indicating that compound 2 is Verdazyl radical.
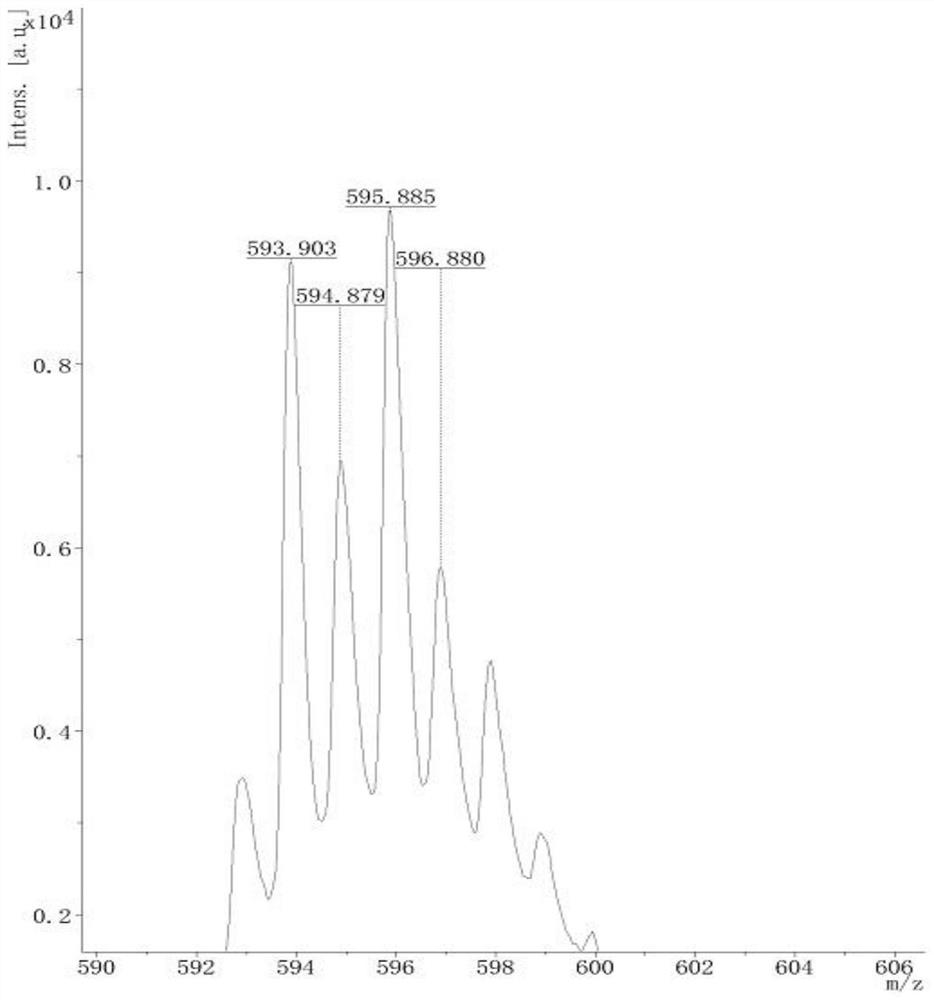
[0115] Figure 7 is the MALDI-TOF diagram of the obtained compound 2, from Figure 7 It can be seen: MALDI-TOF(m / z):[M]calcdfor C 33 H 26 N 5 , 492.61; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com