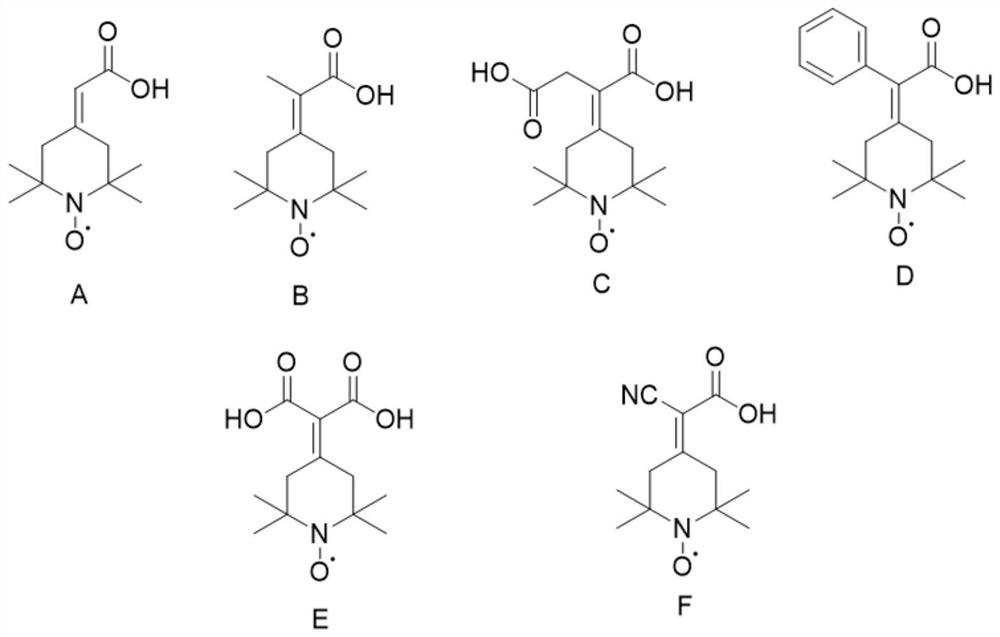
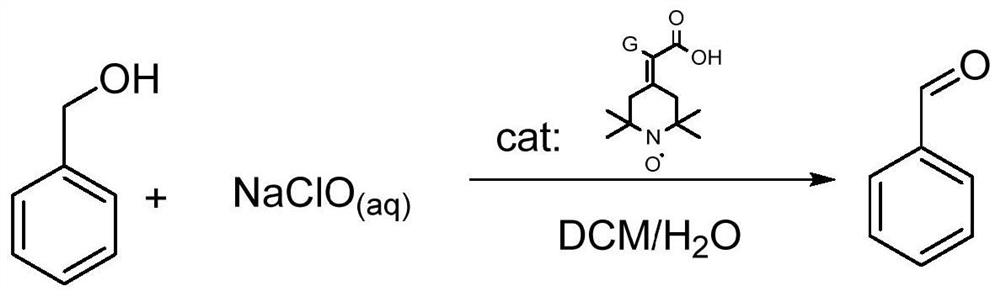
Preparation method of 2-(2, 2, 6, 6-tetramethyl piperidine nitroxide radical-4-subunit) acetic acid derivative and application thereof
A technology of tetramethylpiperidine nitroxide free radicals and derivatives, which is applied in the field of preparation of 2-acetic acid derivatives, can solve the problems of high cost of TEMPO, restrictions on application and development, unstable compounds, etc., and achieve low cost, Easy to obtain, stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
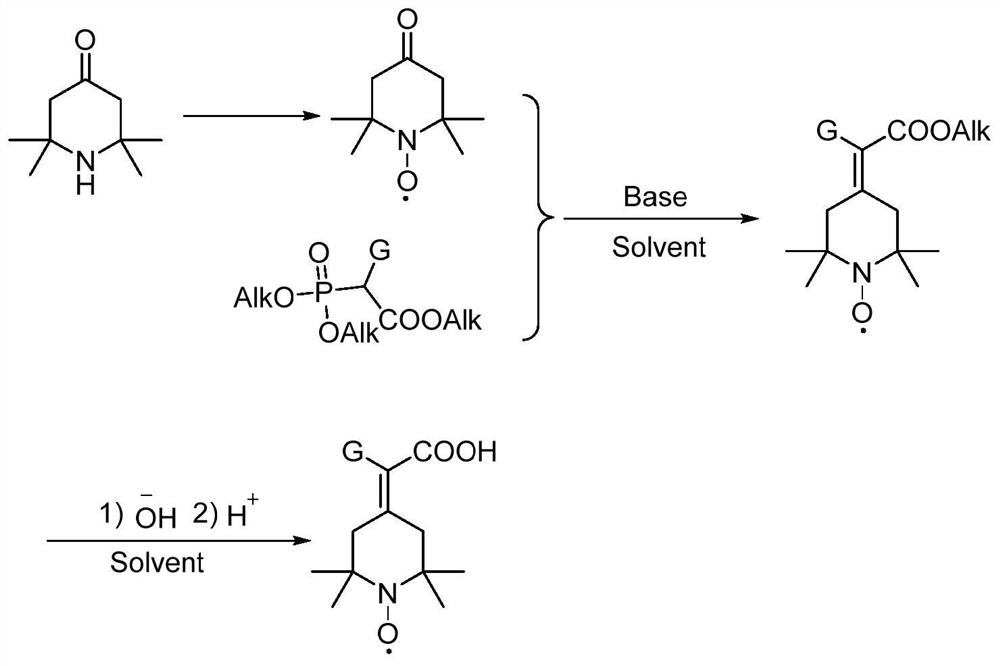
[0036] Example 1: Preparation of 4-carbonyl-2,2,6,6-tetramethylpiperidine nitroxide radical 2,2,6,6-tetramethyl-4-piperidone (50g, 0.322mol , 1eq) was dissolved in a mixture of methanol (300mL) and water (200mL), slowly added dropwise a mixture of sodium tungstate dihydrate (17.6g, 0.055mol, 0.17eq) and hydrogen peroxide (110mL, aq 30%, 3eq), at room temperature Reaction for 8h, TLC detected that the reaction was complete, saturated the aqueous layer with K2CO3, extracted with methyl tert-butyl ether, dried, and spin-dried to give an orange-red solid 4-carbonyl-2,2,6,6-tetramethylpiperidine nitrogen oxide Free radical 48.8g, yield 89%.
Embodiment 2
[0037] Example 2: Preparation of 2-(2,2,6,6-tetramethylpiperidine nitroxide radical-4-ylidene) acetic acid (compound A)
[0038]NaH (3.5g, 88.3mmol, 1.5eq) was added to a 500mL reaction flask, under nitrogen protection, 100mL of dry THF was added, and after stirring at 0°C for 15min, triethyl phosphoroacetate (19.8g, 88.3mmol, 1.5eq), added in about 15 minutes, after the reaction solution in the reaction bottle was clarified and no gas was emitted, add 4-carbonyl-2,2,6,6-tetramethylpiperidine nitroxide radical (10g, 28.7mmol , 1eq), naturally rose to room temperature, and reacted for 4h. After HPLC showed that the reaction of the raw materials was complete, it was quenched with 30mL saturated ammonium chloride solution, extracted with ethyl acetate, and concentrated to obtain a red oily liquid.
[0039] The red oily liquid obtained in the previous step was dissolved in 150mL (MeOH / H 2 (0 / =3 / 1) in the mixed solution, add 4.7g of sodium hydroxide, stir at room temperature for 5...
Embodiment 3
[0040] Example 3: Preparation of 2-(2,2,6,6-tetramethylpiperidinyl nitroxide radical-4-ylidene) propionic acid (compound B)
[0041] The operation process was the same as in Example 2, and the phosphorylating reagent used was triethyl 2-phosphonopropionate to obtain 9.7 g of off-white solid with a purity of 98.1% and a total yield of 73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com