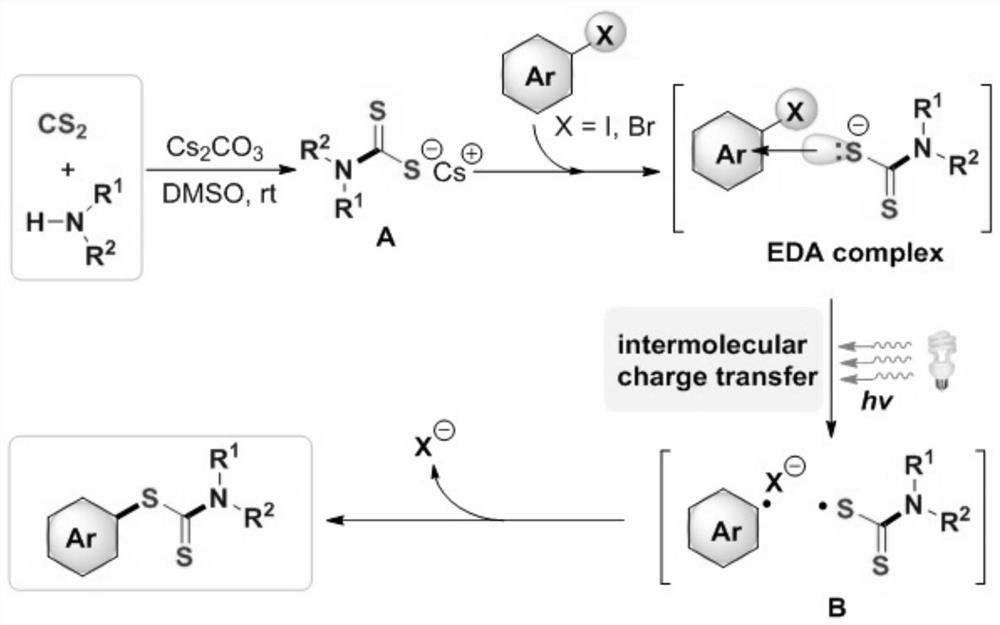
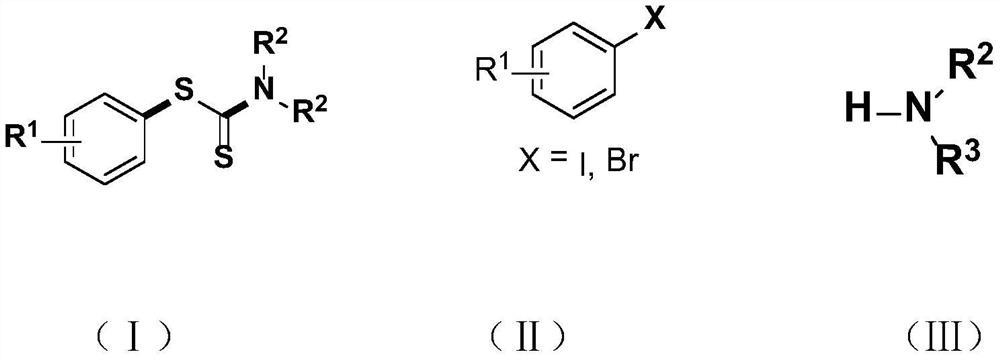
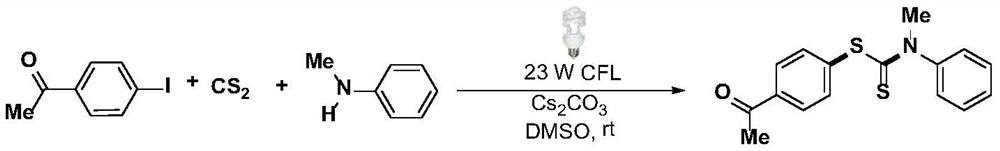
Synthesis method and application of a kind of visible light-promoted dithiocarbamate compound
A technology for dithiocarbamic acid and ester compounds, which is applied in the field of photocatalytic organic synthesis, can solve the problems of inability to meet the screening requirements of active lead compounds, complicated reaction steps, limited methods and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] At room temperature, add the corresponding iodobenzene (0.3mmol), carbon disulfide (0.8mmol), and the corresponding secondary amine (0.4mmol) to a 25ml Schlenk (Schlenk) tube filled with nitrogen and equipped with a magnetic stirring bar. Cesium carbonate (0.6mmol), 2.0mL DMSO was added with a syringe under nitrogen, the reaction tube was placed 3 cm away from a 23-watt fluorescent lamp, irradiated and stirred for 24 hours. After the reaction is over, add 2 mL of deionized water to the reaction liquid, mix well, and use 3 mL of ethyl acetate as the extractant each time to extract the crude product from the reaction liquid through liquid separation and extraction, combine the extracts, and rotate The evaporator was used to remove the solvent; the residue was purified with a silica gel column (the specification of silica gel was 200 mesh to 300 mesh, and the eluent was petroleum ether / ethyl acetate (20:1v / v)) to obtain 70.4 mg of the target product with a yield...
Embodiment 2
[0042]
[0043]At room temperature, add the corresponding iodobenzene (0.3mmol), carbon disulfide (1.2mmol), and the corresponding secondary amine (0.3mmol) to a 25ml Schlenk (Schlenk) tube filled with nitrogen and equipped with a magnetic stirring bar. Potassium carbonate (0.6mmol), 2.0mL DMSO was added with a syringe under nitrogen, and the reaction tube was placed 3 cm away from a 23-watt fluorescent lamp, and irradiated and stirred for 12 hours. After the reaction is over, add 2 mL of deionized water to the reaction solution, mix well, use 3 mL of ethyl acetate as the extractant each time to extract the crude product from the reaction solution, combine the extracts, and rotate The evaporator removed the solvent; the residue was purified with a silica gel column (the specification of silica gel was 200 mesh to 300 mesh, and the eluent was petroleum ether / ethyl acetate (15:1v / v)) to obtain 76.5 mg of the target product, with a yield of 81 %.
[0044] The obtained product...
Embodiment 3
[0049]
[0050] At room temperature, add the corresponding iodobenzene (0.3 mmol), carbon disulfide (0.9 mmol), and the corresponding secondary amine (0.75 mmol) to a 25 ml Schlenk (Schlenk) tube filled with nitrogen and equipped with a magnetic stirring bar, Sodium carbonate (0.9mmol), 2.0mL DMSO was added with a syringe under nitrogen, and the reaction tube was placed 3 cm away from a 23-watt fluorescent lamp, and irradiated and stirred for 22 hours. After the reaction is over, add 2 mL of deionized water to the reaction solution, mix well, use 3 mL of ethyl acetate as the extractant each time to extract the crude product from the reaction solution, combine the extracts, and rotate The evaporator was used to remove the solvent; the residue was purified with a silica gel column (the silica gel specification was 200 mesh to 300 mesh, and the eluent was petroleum ether / ethyl acetate (20:1v / v)) to obtain 58.6 mg of the target product with a yield of 70 %.
[0051] The obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com