Green synthesis method of amino alcohol compounds under visible light catalysis
An amino alcohol, green synthesis technology, applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, organic chemical methods and other directions, can solve the problems of complex experimental steps, low atom economy, etc., to achieve simple synthesis route and reaction conditions. Good controllability and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
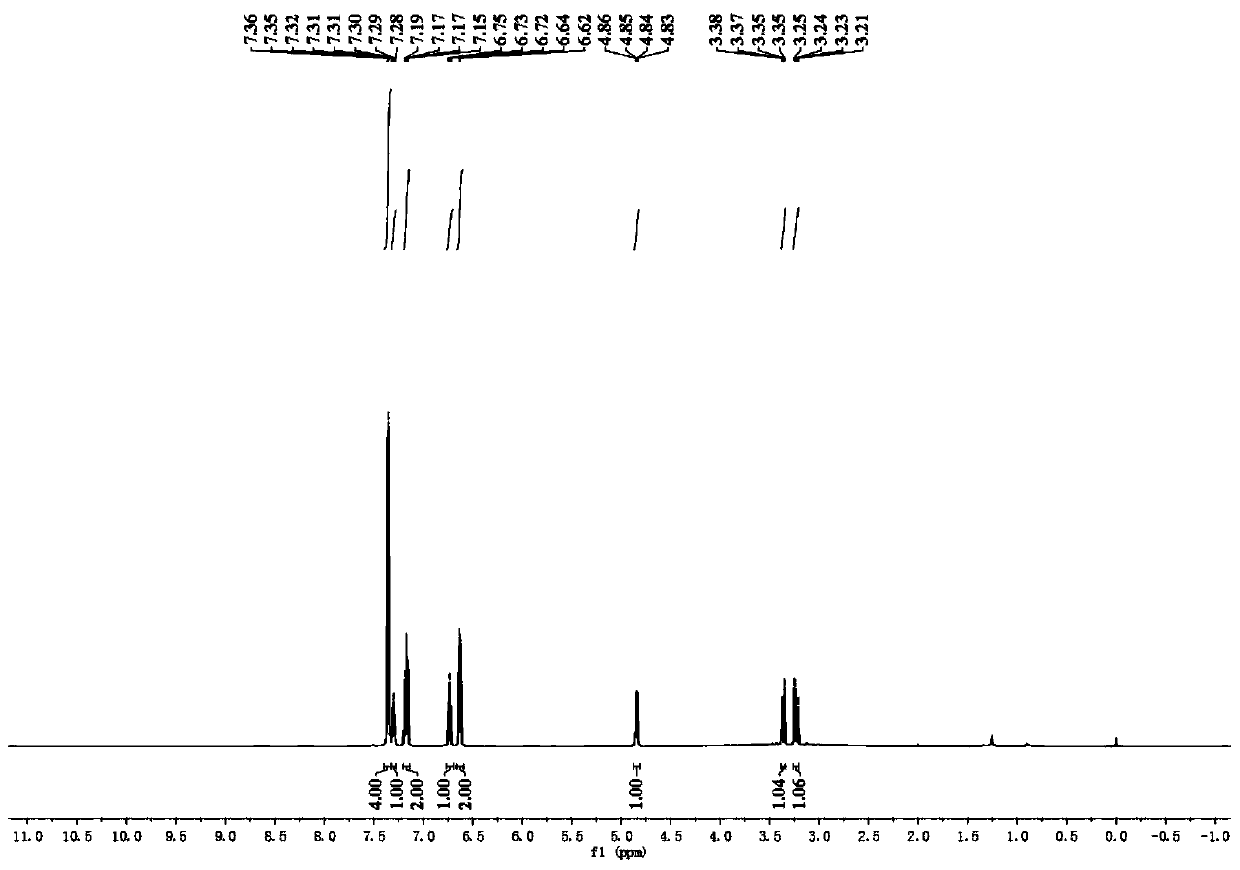
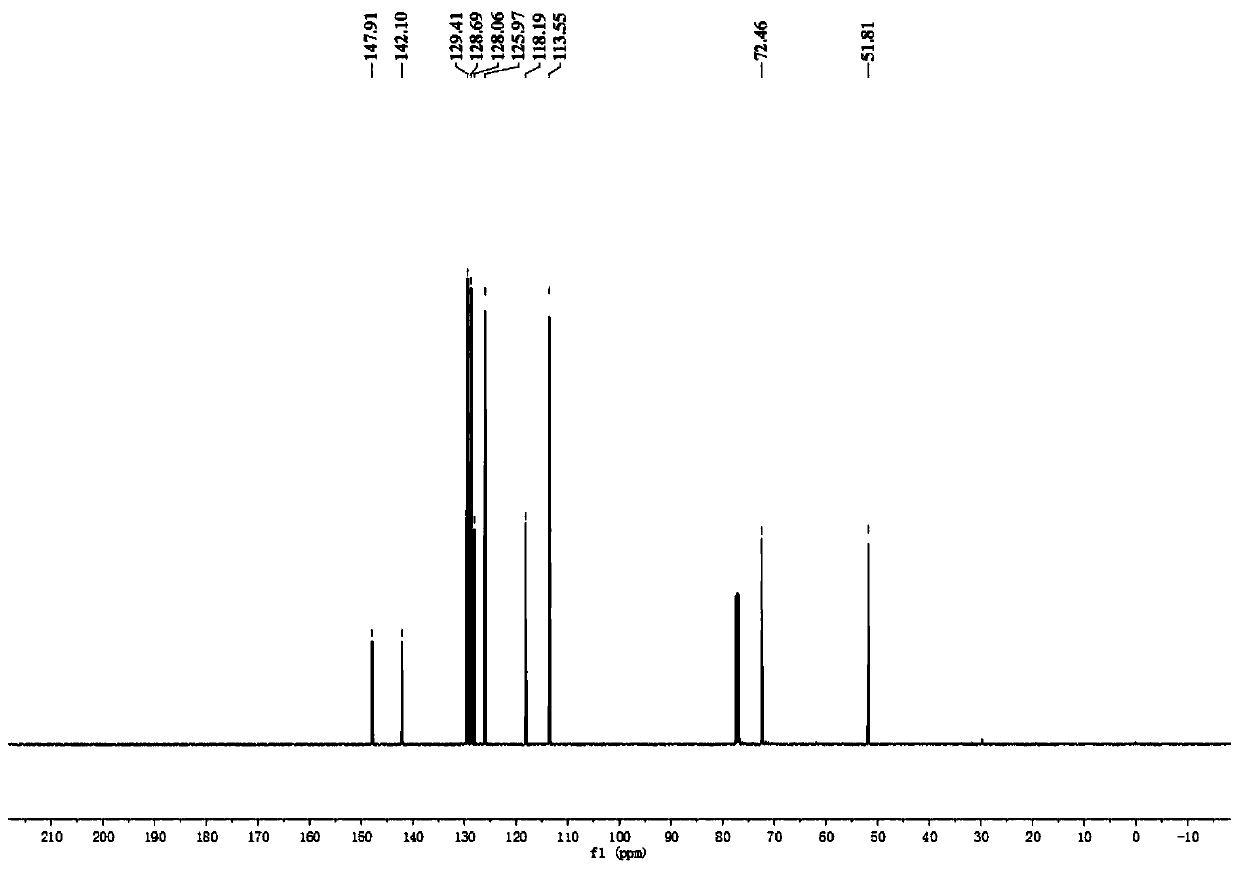
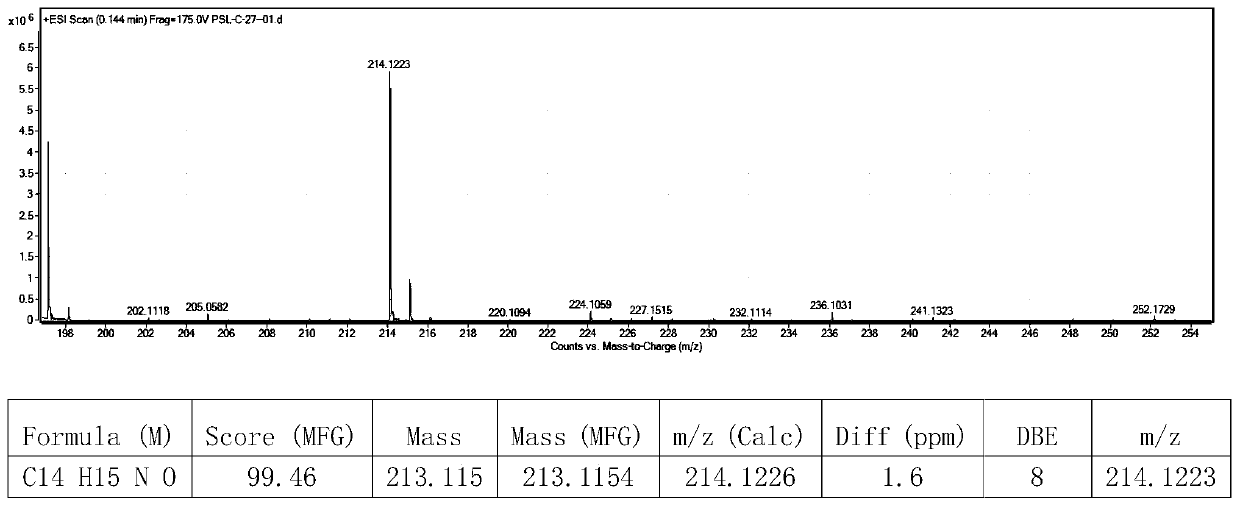
[0074] In a dry 10ml Schlenk tube, add 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2ml of water, 20.4 μl of benzaldehyde, the ratio of benzaldehyde: N-phenylglycine: photocatalyst is 1:3:0.01. The reaction bottle was pumped and ventilated three times to ensure that the reaction tube was anhydrous and oxygen-free, then sealed, and the reaction was placed under the irradiation of 455nm blue light (Blue LED light) and stirred for 4 hours. The distance between the Blue LED light and the Schlenk tube was 3cm. After the reaction was detected by TLC, it was extracted 4 times with 5ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Drying, adding an appropriate amount of silica gel and concentrating under reduced pressure, and the resulting residue was purified by column chromatography (PE / EA=5:1), as Figure 1-3 As shown, the synthesized product was indeed N-phenylphenylethanolamine through qualitative...
Embodiment 2
[0080] In a dry 10ml Schlenk tube, add 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2ml of water, 24.0 μl of p-methoxybenzaldehyde, p-methoxybenzaldehyde: N-phenylglycine: photocatalyst in a ratio of 1:3:0.01. The reaction bottle was pumped and ventilated three times to ensure that the reaction tube was anhydrous and oxygen-free, then sealed, and the reaction was placed under 455nm blue light (Blue LED light) and stirred for 5 hours. The distance between the Blue LED light and the Schlenk tube was 3cm. After the reaction was detected by TLC, it was extracted 4 times with 5ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the resulting residue is purified by column chromatography (PE / EA=4:1), as Figure 4-6 Shown, the synthetic product by qualitative and quantitative analysis is indeed 1-(4-methoxyphenyl)-2-(...
Embodiment 3
[0082] In a dry 10ml Schlenk tube, add 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2ml of water, 22.8 μl of p-tolualdehyde, the ratio of p-tolualdehyde: N-phenylglycine: photocatalyst is 1:3:0.01. The reaction bottle was pumped and ventilated three times to ensure that there was no water and oxygen in the reaction tube, and then sealed, and the reaction was placed under the irradiation of 455nm blue light (Blue LED lamp) and stirred for 2h. After the reaction was detected by TLC, it was extracted 4 times with 5ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the resulting residue is purified by column chromatography (PE / EA=4:1), as Figure 7-9Shown, the synthetic product by qualitative and quantitative analysis is indeed 1-(p-methylphenyl)-2-(phenylamino)-ethan-1-alcohol, obtains product 1-(p-methylphenyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com