Synthesis method of (E)-1-styrylcyclohexane-1-nitrile compound
The technology of a styryl group and a synthesis method is applied in the synthesis field of alkene nitrile compounds, can solve the problems of low product yield, low atom utilization rate, unfriendly environment, etc., achieves high atom utilization rate and avoids waste of metal catalysts the effect of avoiding the use of toxic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
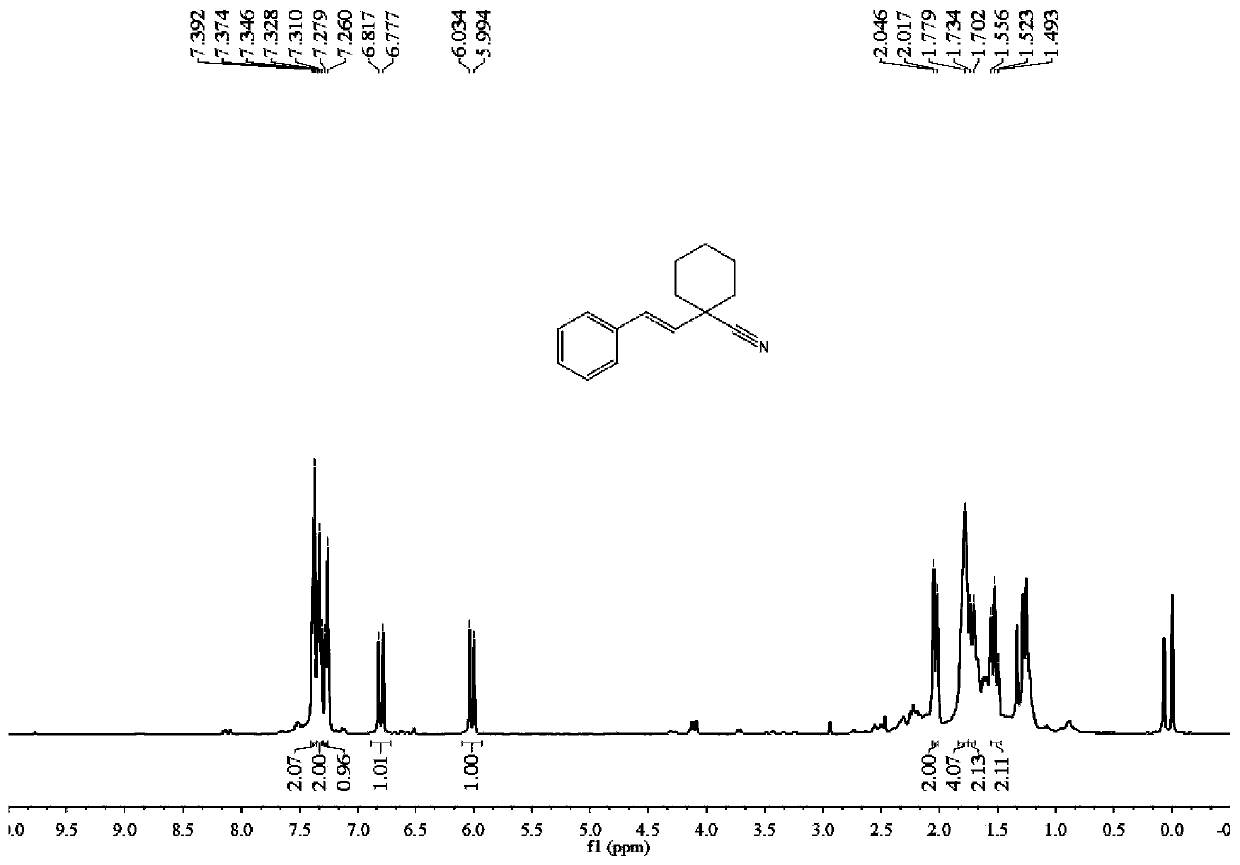
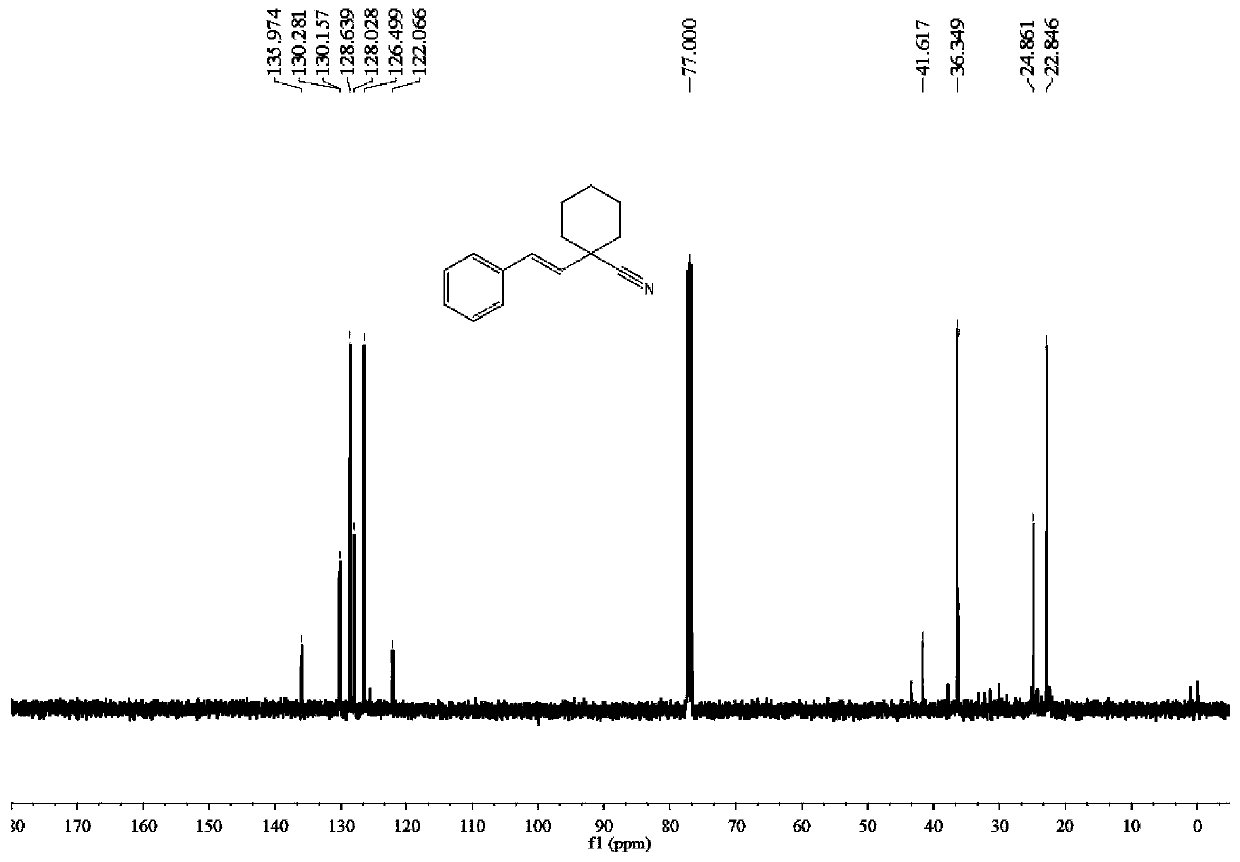
[0046] Raw materials: styrene;
[0047] Product: (E)-1-styrylcyclohexane-1-carbonitrile
[0048]
[0049] Eluent PE / EA=60:1. 87mg of light yellow oily liquid product was obtained, the yield was 82%.
[0050] 1 H NMR (400MHz, CDCl 3 )δ7.38(d, J=7.3Hz, 2H), 7.33(t, J=7.1Hz, 2H), 7.27(d, J=7.6Hz, 1H), 6.80(d, J=16.0Hz, 1H) ,6.01(d,J=16.0Hz,1H),2.03(d,J=11.9Hz,2H),1.85–1.67(m,4H),1.72(d,J=12.9Hz,2H),1.52(t, J=12.5Hz, 2H). 13 C NMR (101MHz, CDCl 3 )δ135.97, 130.28, 130.16, 128.64, 128.03, 126.50, 122.07, 41.62, 36.35, 24.86, 22.85. m / z=211.
Embodiment 2
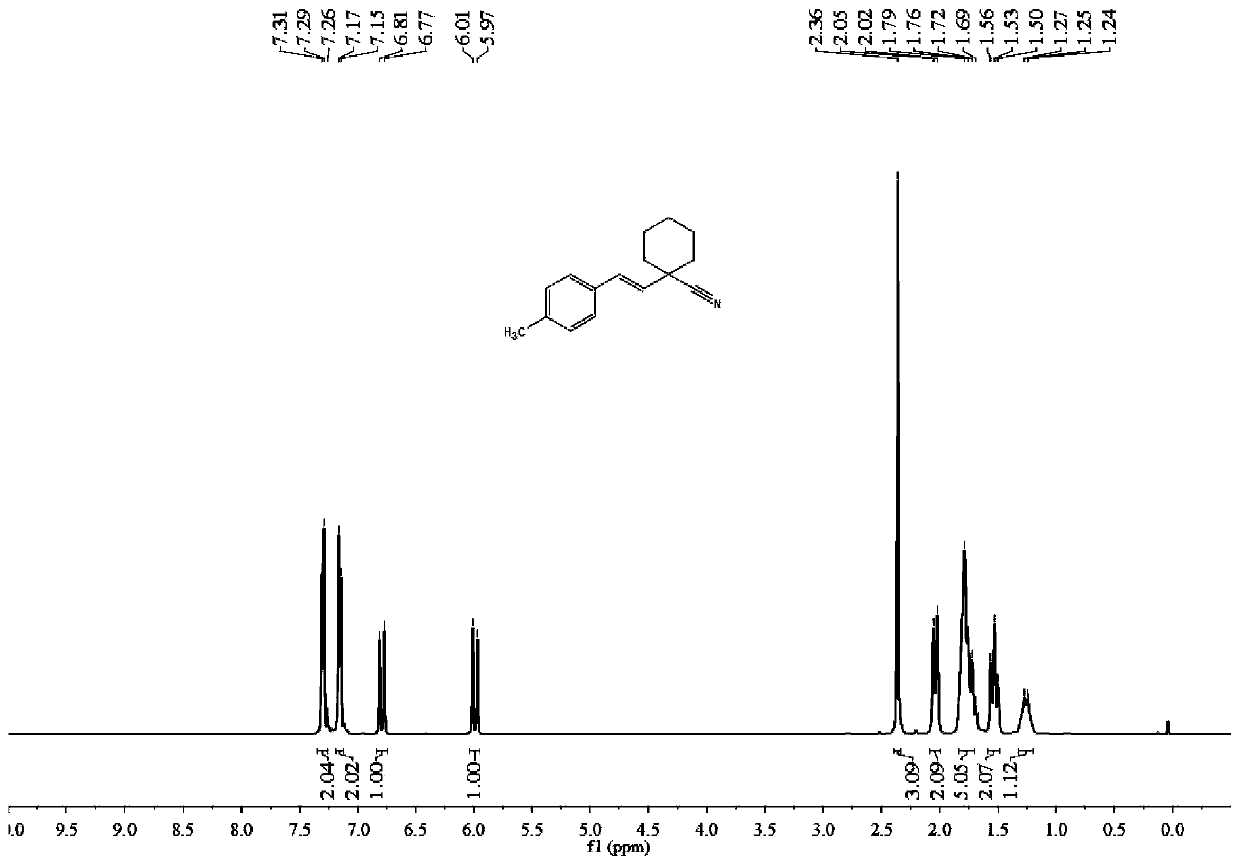
[0052] Raw material: 4-methylstyrene;
[0053] Product: (E)-1-(4-methylstyryl)cyclohexane-1-carbonitrile
[0054]
[0055] Eluent PE / EA=60:1. 94 mg of bright yellow oily liquid product was obtained, with a yield of 84%.
[0056] 1 H NMR (400MHz, CDCl 3 )δ7.30(d, J=7.6Hz, 2H), 7.16(d, J=7.6Hz, 2H), 6.79(d, J=16.0Hz, 1H), 5.99(d, J=16.0Hz, 1H) ,2.36(s,3H),2.04(d,J=13.1Hz,2H),1.74(dd,J=26.8,13.2Hz,5H),1.53(t,J=12.3Hz,2H),1.40–1.06( m,1H). 13 C NMR (101MHz, CDCl 3 )δ137.76, 133.08, 129.85, 129.18 (d, J=3.8Hz), 126.28, 122.01, 41.42, 36.25, 24.75, 22.75, 21.04.m / z=225.
Embodiment 3
[0058] Raw material: 4-chlorostyrene;
[0059] Product: (E)-1-(4-chlorostyryl)cyclohexane-1-carbonitrile
[0060]
[0061] Eluent PE / EA=60:1. 106 mg of dark yellow oily liquid product was obtained with a yield of 87%.
[0062] 1 H NMR (400MHz, CDCl 3 )δ7.29(s,4H),6.75(d,J=16.0Hz,1H),5.99(d,J=16.0Hz,1H),2.02(d,J=13.1Hz,2H),1.87–1.62( m,5H), 1.52(t,J=12.5Hz,2H),1.35–0.97(m,1H). 13 C NMR (101MHz, CDCl 3 )δ134.47, 133.67, 130.93, 129.00, 128.76, 127.70, 121.83, 41.62, 36.28, 24.81, 22.80. m / z=245.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com