Conjugated double-free-radical molecule as well as preparation method, spin distribution regulation and control method and application thereof
A free radical and conjugation technology, applied in the production of organic free radicals, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of difficulty in regulating the distribution of free radical spins, complicated synthesis of conjugated biradical molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A kind of conjugated diradical molecule, its structure is as shown in formula (I),
[0036]
[0037] The conjugated diradical molecule in this example is prepared through the following steps:
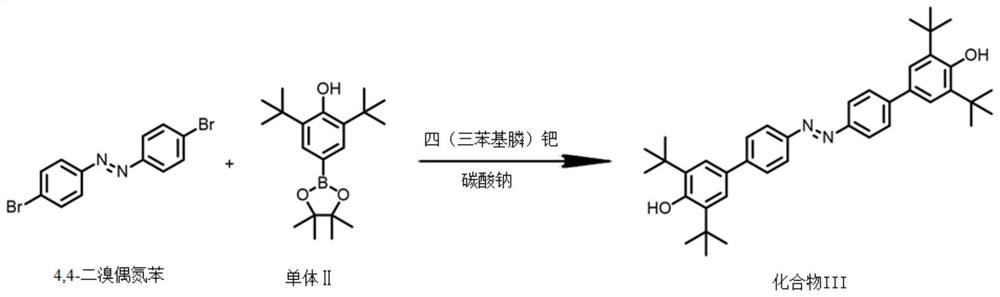
[0038] S1: Mix 4,4-dibromoazobenzene, monomer II, tetrakis(triphenylphosphine) palladium and sodium carbonate at a molar ratio of 1:2.5:0.075:0.2, and store at 120°C in an inert gas atmosphere Condensate and reflux in a mixed solvent of tetrahydrofuran and water for 12 hours to obtain compound III. The volume ratio of water and tetrahydrofuran in the mixed solvent of tetrahydrofuran and water is 1:2; the reaction equation is as follows figure 1 shown;
[0039] S2: Dissolve compound III in dichloromethane, add lead dioxide, and react at room temperature for 30 minutes to obtain conjugated diradical molecules; the molar ratio of compound III to lead dioxide is 1:50.
Embodiment 2
[0041] A kind of conjugated diradical molecule, its structure is as shown in formula (I),
[0042]
[0043] The conjugated diradical molecule in this example is prepared through the following steps:
[0044] S1: Mix 4,4-dibromoazobenzene, monomer II, tetrakis(triphenylphosphine) palladium and sodium carbonate at a molar ratio of 1:3:0.08:0.4, and store at 115°C in an inert gas atmosphere Condensation and reflux reaction in a mixed solvent of tetrahydrofuran and water for 15h to obtain compound III, the volume ratio of water and tetrahydrofuran in the mixed solvent of tetrahydrofuran and water is 1:2; the reaction equation is as follows figure 1 shown;
[0045] S2: Dissolve compound III in dichloromethane, add lead dioxide, and react at room temperature for 25 minutes to obtain conjugated diradical molecules; the molar ratio of compound III to lead dioxide is 1:55.
Embodiment 3
[0047] A kind of conjugated diradical molecule, its structure is as shown in formula (I),
[0048]
[0049] The conjugated diradical molecule in this example is prepared through the following steps:
[0050] S1: Mix 4,4-dibromoazobenzene, monomer II, tetrakis(triphenylphosphine)palladium and sodium carbonate at a molar ratio of 1:2:0.06:0.2, and store at 125°C in an inert gas atmosphere Condensate and reflux in a mixed solvent of tetrahydrofuran and water for 12 hours to obtain compound III. The volume ratio of water and tetrahydrofuran in the mixed solvent of tetrahydrofuran and water is 1:2; the reaction equation is as follows figure 1 shown;
[0051] S2: Dissolve compound III in dichloromethane, add lead dioxide, and react at room temperature for 35 minutes to obtain conjugated diradical molecules; the molar ratio of compound III to lead dioxide is 1:40.
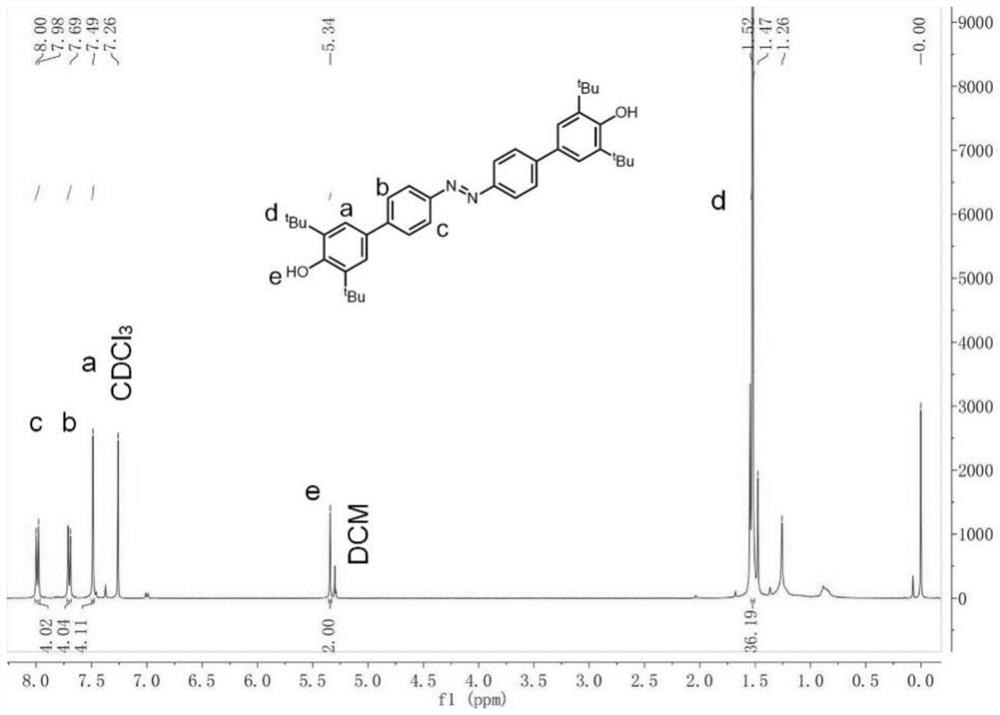
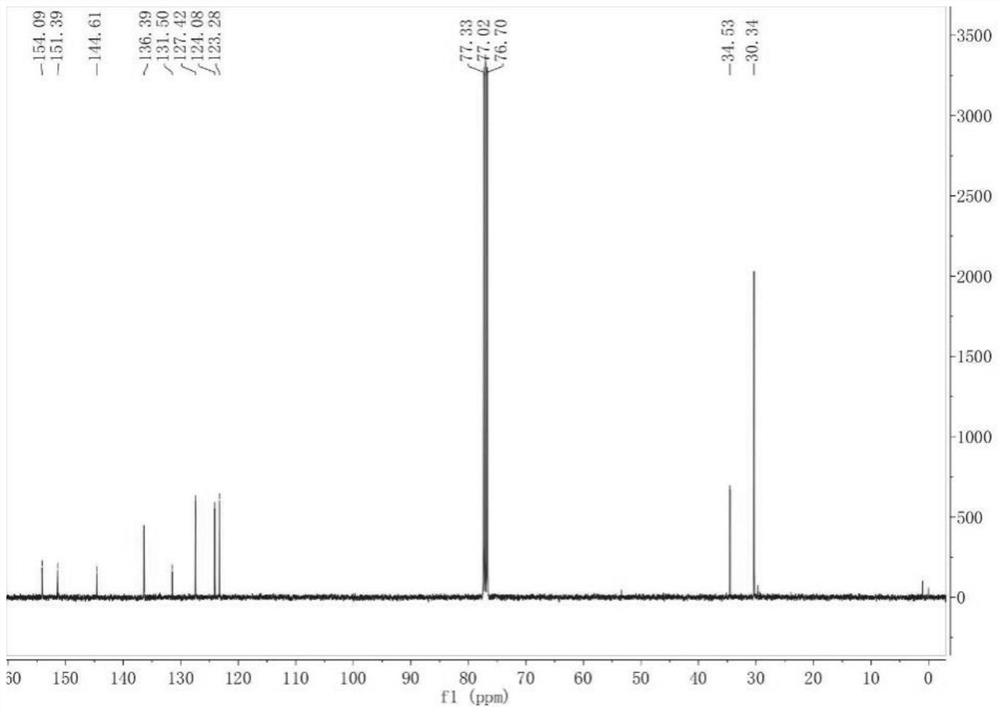
[0052] Result analysis
[0053] Taking Example 1 as an example, the compound III it makes carries out nuclear mag...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com