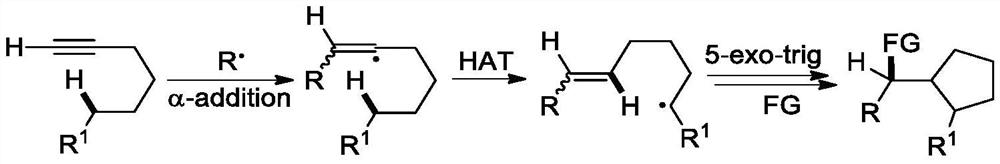
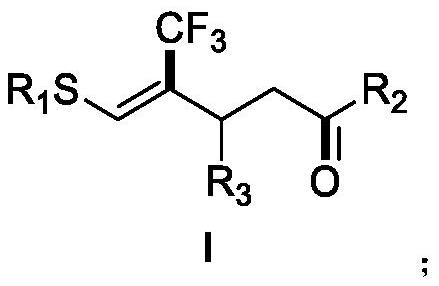
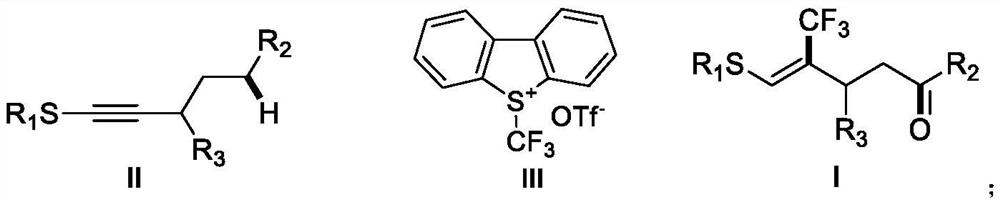
A kind of (z)-4-trifluoromethyl-5-sulfanyl-4-pentenone derivative and its preparation method
A technology of trifluoromethyl and thienium trifluoromethanesulfonate, applied in the field of -4-trifluoromethyl-5-sulfanyl-4-pentenone derivatives and their preparation, can solve the problem of There are no problems such as olefin addition products, and the effect of wide application range of substrates, good functional group compatibility and simple operation is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take a dry reaction tube, add tris(2,2-bipyridyl)ruthenium dichloride (3.0mg, 0.004mmol), sodium bicarbonate (33.6mg, 0.4mmol), S shown in structural formula 2 under nitrogen atmosphere -(Trifluoromethyl)dibenzothiophenium trifluoromethanesulfonate (160.0mg, 0.40mmol), alkyne thioether (38mg, 0.2mmol) represented by structural formula 1a was then added to 2 mL of dry dimethylmethylene Sulfone forming reaction system. Under the irradiation of 20W blue light, the system was stirred at room temperature 25°C for 8h, quenched by adding 15mL of water, extracted three times with ethyl acetate (10mL), combined, the organic phase was washed with saturated edible water, and dried over anhydrous sodium sulfate. After the organic phase was concentrated, it was separated by silica gel (300-400 mesh) column chromatography to obtain 41 mg of a light yellow liquid represented by structural formula 3a, with a yield of 75%.
[0031] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3 )δ...
Embodiment 2
[0035] Except that the alkyne thioether derivative shown in structural formula 1b was used instead of the alkyne thioether derivative shown in structural formula 1a in Example 1, the rest of the operation steps were the same as in Example 1, yield: 73%, light yellow liquid shown in structural formula 3b.
[0036] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3)δ7.95–7.94(m,2H),7.59–7.56(m,1H),7.48–7.46(m,2H),6.53(s,1H),3.16(t,J=7.4Hz,2H),2.73 (q, J=7.4Hz, 2H), 2.71–2.67(m, 2H), 1.31(t, J=7.4Hz, 3H); 13 C NMR (151MHz, CDCl 3 )δ198.5, 136.7, 135.5(q, J=3.2Hz), 133.2, 128.6, 127.9, 124.6(q, J=275.1Hz), 122.8(q, J=29.9Hz), 37.7, 29.3(q, J=1.9 Hz), 27.1 (q, J=1.7Hz), 15.3; 19 F NMR (565MHz, CDCl 3 )δ-61.5; HRMS (ESI) calcd for C 14 h 15 f 3 OSNa(M+Na) + 311.0688, found 311.0690.
[0037] The reaction formula is as follows:
[0038]
Embodiment 3
[0040] Except that the alkyne thioether derivative represented by structural formula 1c was used to replace the alkyne thioether represented by structural formula 1a in Example 1, the remaining operating steps were the same as in Example 1, yield: 76%, light yellow liquid represented by structural formula 3c.
[0041] Product Spectrum Analysis 1 H NMR (600MHz, CDCl 3 )δ7.85(d, J=8.1Hz, 2H), 7.26(d, J=8.0Hz, 2H), 6.46(s, 1H), 3.13(t, J=7.5Hz, 2H), 2.66(t, J=7.5Hz, 2H), 2.41(s, 3H), 2.34(s, 3H); 13 C NMR (151MHz, CDCl 3 )δ198.1, 144.1, 137.2(q, J=3.2Hz), 134.2, 129.3, 128.1, 124.6(q, J=275.1Hz), 122.7(q, J=29.8Hz), 37.5, 27.0(q, J=1.8 Hz), 21.6, 18.4 (q, J=2.1Hz); 19 F NMR (565MHz, CDCl 3 )δ-61.5; HRMS (ESI) calcd for C 14 h 15 f 3 OSNa(M+Na) + 311.0688,found 311.0689.
[0042] The reaction formula is as follows:
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com