Green Synthesis of Amino Alcohols Catalyzed by Visible Light
A green synthesis technology of amino alcohols, which is applied in the preparation of amino hydroxyl compounds, the preparation of organic compounds, organic chemical methods, etc., can solve the problems of complex experimental steps and low atom economy, and achieve simple synthetic routes and reliable reaction conditions. Good controllability and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
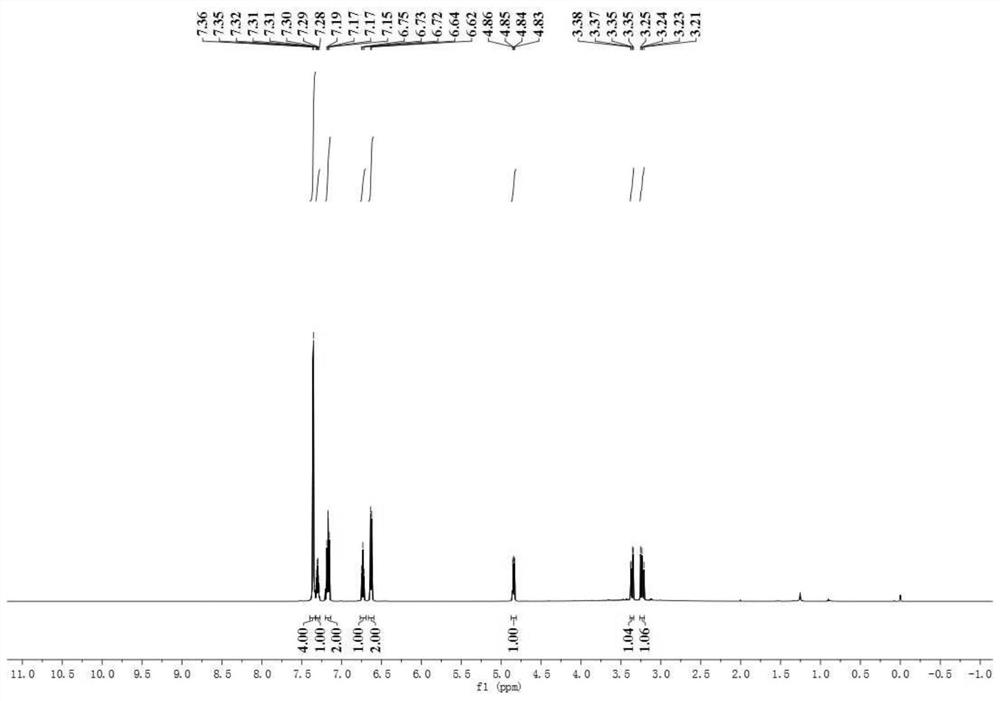
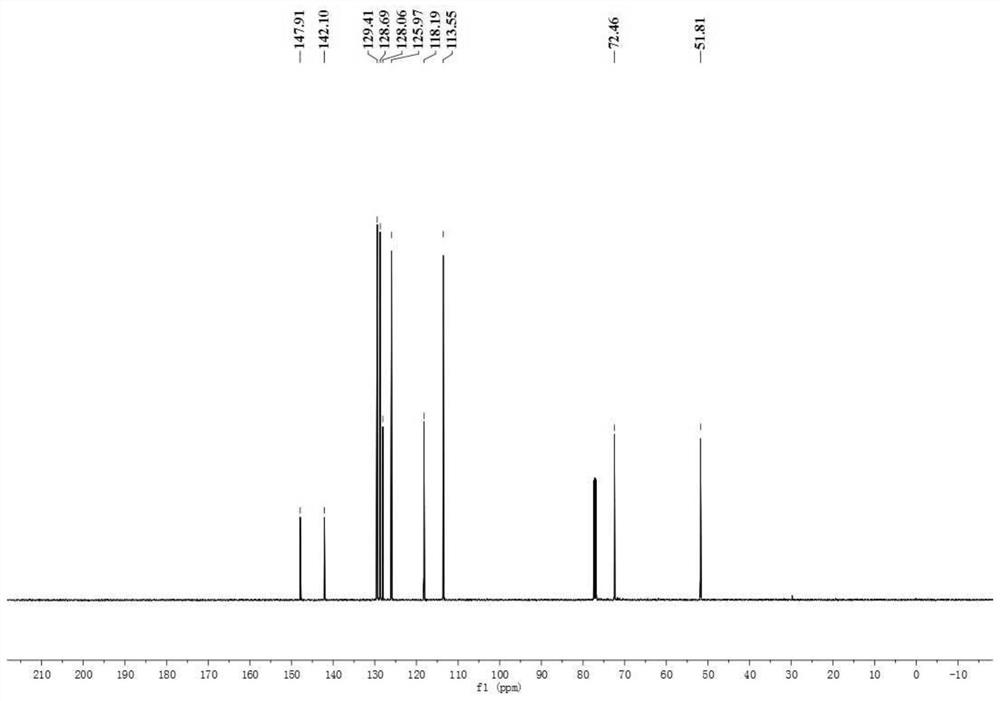
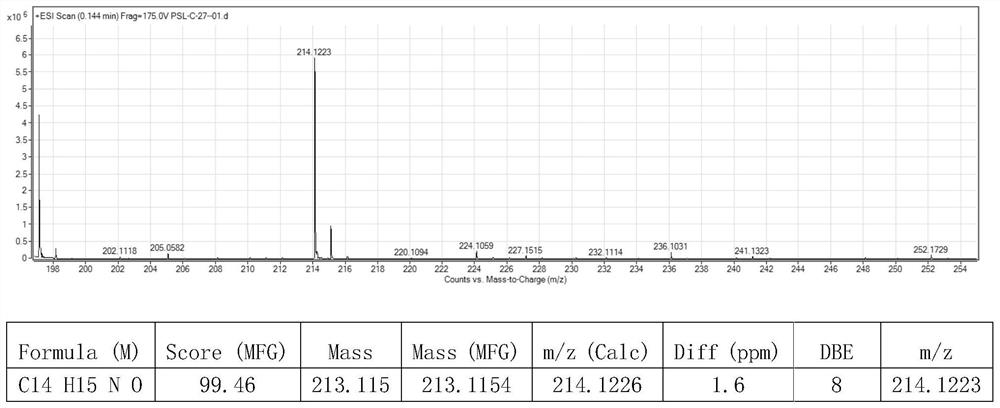
[0074] In a dry 10ml Schlenk tube, 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2 ml of water, 20.4 μl of benzaldehyde, and the amount of benzaldehyde: N-phenylglycine: photocatalyst is 1:3:0.01. The reaction flask was pumped and ventilated three times to ensure that there was no water and oxygen in the reaction tube and then sealed, and the reaction was placed under the irradiation of 455 nm blue light (Blue LED lamp) and stirred for 4 h. The distance between the Blue LED lamp and the Schlenk tube was 3 cm. After the reaction was detected by TLC, it was extracted 4 times with 5 ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the obtained residue is purified by column chromatography (PE / EA=5:1), as Figure 1-3 As shown, the synthesized product was indeed N-phenylphenethanolamine by qualitative and quantita...
Embodiment 2
[0080] In a dry 10ml Schlenk tube, 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2 ml of water, 24.0 μl of p-methoxybenzaldehyde, and the ratio of the amount of p-methoxybenzaldehyde: N-phenylglycine: photocatalyst is 1:3:0.01. The reaction flask was pumped and ventilated three times to ensure that there was no water and oxygen in the reaction tube and then sealed, and the reaction was placed under the irradiation of 455 nm blue light (Blue LED lamp) and stirred for 5 h. The distance between the Blue LED lamp and the Schlenk tube was 3 cm. After the reaction was detected by TLC, it was extracted 4 times with 5 ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the obtained residue is purified by column chromatography (PE / EA=4:1), as Figure 4-6 As shown, the synthesized product by qualitative and quantitative ...
Embodiment 3
[0082] In a dry 10ml Schlenk tube, 90.4mg N-phenylglycine, 1.9mg photocatalyst [Ir(ppy) 2 dtbbpy]PF 6 , 2 ml of water, 22.8 μl of p-toluidine, and the ratio of p-tolualdehyde:N-phenylglycine:photocatalyst is 1:3:0.01. The reaction flask was pumped and ventilated three times to ensure that there was no water and oxygen in the reaction tube, and then the reaction tube was sealed, and the reaction was placed under the irradiation of 455 nm blue light (Blue LED lamp) and stirred for 2 h. After the reaction was detected by TLC, it was extracted 4 times with 5 ml of ethyl acetate respectively, and the organic phase was collected and washed with anhydrous Na 2 SO 4 Dry, add an appropriate amount of silica gel and concentrate under reduced pressure, and the obtained residue is purified by column chromatography (PE / EA=4:1), as Figure 7-9As shown, the synthesized product by qualitative and quantitative analysis is indeed 1-(p-methylphenyl)-2-(phenylamino)-ethan-1-ol, and the product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com