A kind of synthetic method of α-position cycloalkyl substituted ketone compound
A technology of ketone compounds and synthetic methods, which is applied in the direction of carbon-based compound preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problems of low reactivity of enamine intermediates and affect practical value, etc., and achieve short reaction time , Improved atom economy, high effect of atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
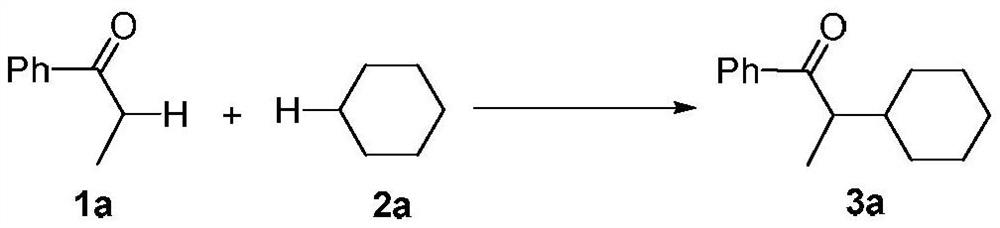
[0012]
[0013] Add propiophenone (1a, 0.5mmol, 67mg), cyclohexane (2a, 5mL) and di-tert-butyl peroxide (2mmol, 380μL) into a 25mL pressure-resistant tube, vacuumize and fill with nitrogen, and then put the pressure-resistant tube Seal it and place it in a microwave reactor, and stir and react at 160° C. for 1 hour. After the reaction, the unreacted cyclohexane was recovered by distillation under reduced pressure, and the residue was separated through a silica gel column (petroleum ether / dichloromethane=6 / 1) to obtain a colorless oily product 3a (67 mg, 62%). The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 )δ:0.95-1.11(m,2H),1.15(d,J=7.2Hz,3H),1.17-1.27(m,3H),1.63-1.68(m,3H),1.73-1.77(m,3H) ,3.33(quint,J=6.8Hz,1H),7.46-7.50(m,2H),7.55-7.57(m,1H),7.95-7.97(m,2H). 13 C NMR (100MHz, CDCl 3 )δ: 14.0, 26.3, 26.4, 26.5, 29.2, 32.1, 40.7, 46.1, 128.2, 128.6, 132.8, 137.5, 205.1. HRMS calcd for C 15 h 21 O:217.1587[M+H] + ,found: 217.1589. ...
Embodiment 2
[0015] Add propiophenone (1a, 0.5mmol, 67mg), cyclohexane (2a, 5mL) and di-tert-butyl peroxide (1mmol, 190μL) into a 25mL pressure-resistant tube, vacuumize and fill with nitrogen, and then put the pressure-resistant tube Seal it and place it in a microwave reactor, and stir and react at 140° C. for 1 hour. After the reaction, the unreacted cyclohexane was recovered by distillation under reduced pressure, and the residue was separated through a silica gel column (petroleum ether / dichloromethane=6 / 1) to obtain a colorless oily product 3a (28 mg, 26%).
Embodiment 3
[0017] Add propiophenone (1a, 0.5mmol, 67mg), cyclohexane (2a, 5mL) and di-tert-butyl peroxide (1mmol, 190μL) into a 25mL pressure-resistant tube, vacuumize and fill with nitrogen, and then put the pressure-resistant tube Seal it and place it in a microwave reactor, and stir and react at 160° C. for 1 hour. After the reaction, the unreacted cyclohexane was recovered by distillation under reduced pressure, and the residue was separated through a silica gel column (petroleum ether / dichloromethane=6 / 1) to obtain a colorless oily product 3a (37 mg, 34%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com