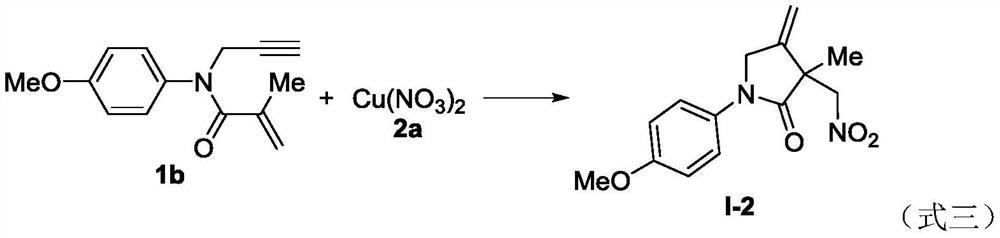
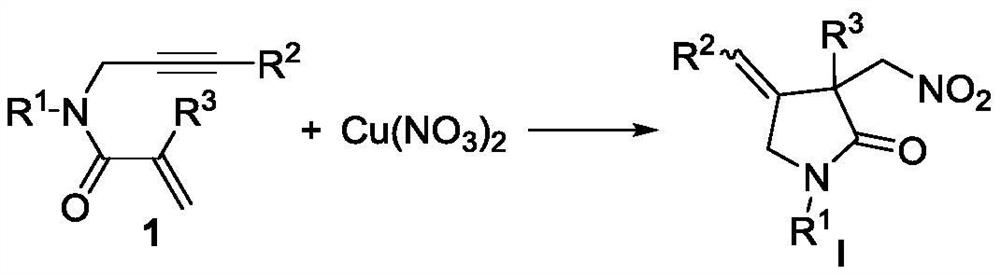A new method based on nitration/cyclization of 1,6-enynes
A cyclization reaction and compound technology, applied in chemical instruments and methods, organic radical generation, organic chemistry, etc., can solve the problems of low reaction atom economy, uncontrollable reaction system, low regioselectivity, etc., and achieve good Application prospect, low cost, and the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
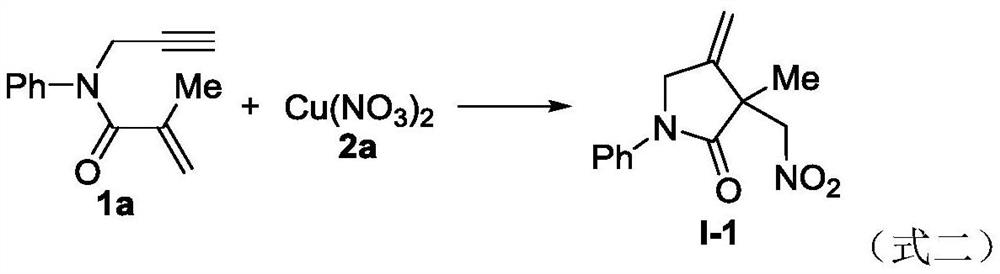
[0029] Add 1,6-enyne compound (39.8mg, 0.2mmol) shown in formula 1a, copper nitrate (74.8mg, 0.4mmol) shown in formula 2a, potassium persulfate (K 2 S2 o 8 , 64.8mg, 0.24mmol) and N,N-dimethylformamide (2mL), then the reactor was stirred and reacted in an air atmosphere at 80°C, and the reaction progress was monitored by TLC until the raw materials disappeared (the reaction time was 4 hours ), after the reaction was completed, the reaction solution was extracted with ethyl acetate into the organic phase, then the obtained organic phase was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to remove the solvent, the residue was separated by column chromatography, column chromatography The elution solvent in is: ethyl acetate / n-hexane to obtain the target product I-1 (90% yield); 1 H NMR (500MHz, CDCl 3 )δ: 7.68(d, J=8.0Hz, 2H), 7.40(t, J=8.0Hz, 2H), 7.20(t, J=7.5Hz, 1H), 5.30(t, J=2.0Hz, 1H) ,5.20(t,J=3.5Hz,1H),4.97(d,J=14.0Hz,...
Embodiment 2
[0031] No oxidizing agent potassium persulfate was added, other conditions were the same as in Example 1, and the yield of the target product I-1 was 0%.
Embodiment 3
[0033] Iodobenzene acetate (PhI(OAc) 2 , 77.3mg, 0.24mmol) instead of potassium persulfate, all the other conditions were the same as in Example 1, and the yield of the target product I-1 was 21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com