Method for synthesizing sophocarpine
A synthesis method and technology of sophocarpine can be applied in the direction of organic chemistry, etc., and can solve the problems such as difficulty in purifying sophocarpine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
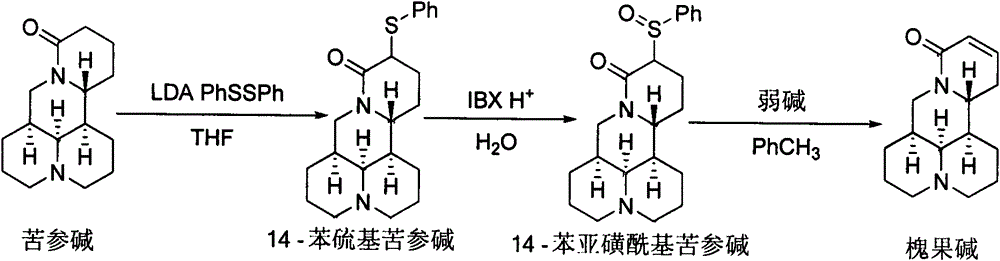
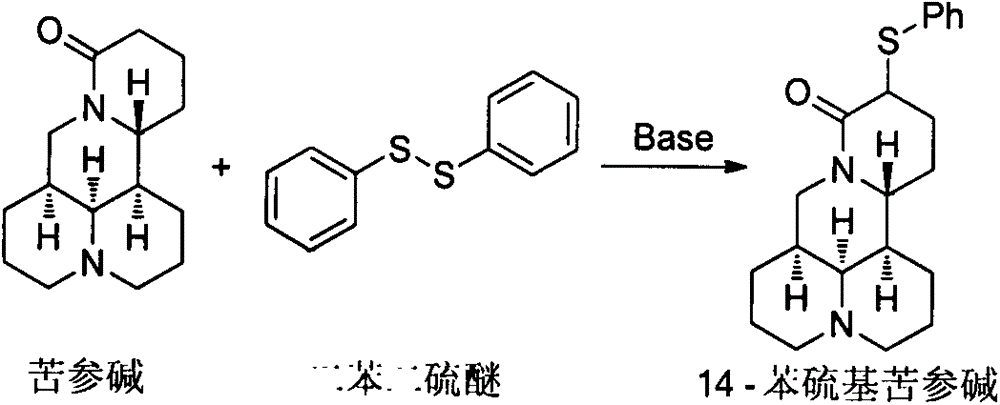
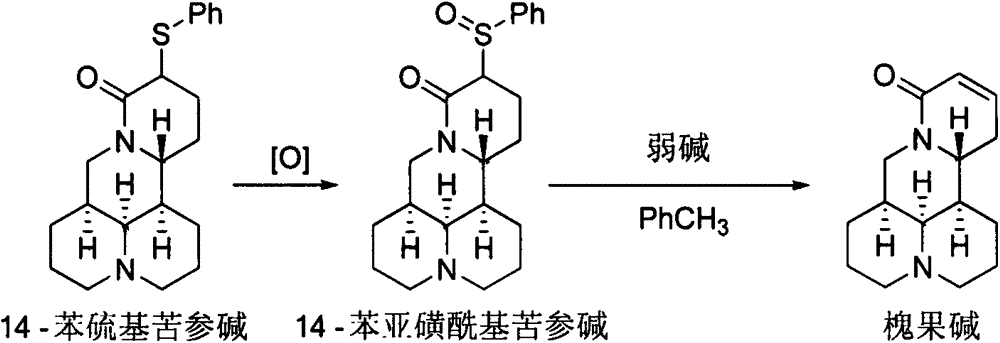
[0007] Synthesis of 14-phenylthiomatrine:
[0008]
[0009] Add 70 mL of tetrahydrofuran into a 250 mL four-neck flask, and lower the temperature to -78°C under the protection of argon. Add 5.0mL (35.6mmol) diisopropylamine and 16.1mL (38.9mmol) n-hexane solution (2.4mol / L) of n-butyllithium to the flask, stir for 15min, then add 4.0g (16.1mmol) Sophora flavescens to the flask Alkali and 3.6g (16.4mmol) diphenyl disulfide tetrahydrofuran solution (30mL), warmed up to room temperature, after stirring for 2h, add 50mL saturated sodium carbonate solution to the system, separate the liquids, the water phase is thoroughly washed with ethyl acetate several times. Extract, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, remove the solvent, and separate by silica gel column chromatography to obtain 5.5 g of yellow liquid. Yield: 95.8%. 1 H NMR (400MHz, CDCl 3 )δ7.56-7.47(m, 2H), 7.32-7.22(m, 3H), 4.39(dd, J=12.8, 4.4Hz, 1H), 3.91-3.76(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com