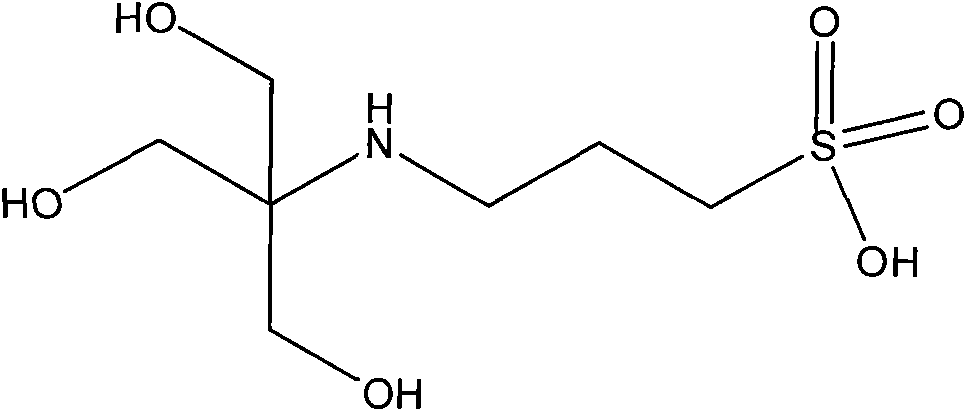
N-tris (hydroxymethyl) methyl-3-aminopropanesulfonic acid compound and preparation method thereof
A technology of trimethylolaminomethane and aminopropanesulfonic acid, which is applied in the field of N-trimethyl-3-aminopropanesulfonic acid compound and its preparation, can solve the problems of low yield, few synthesis processes and the like, and achieves high yield. The effect of high yield, easy availability of raw materials and few synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
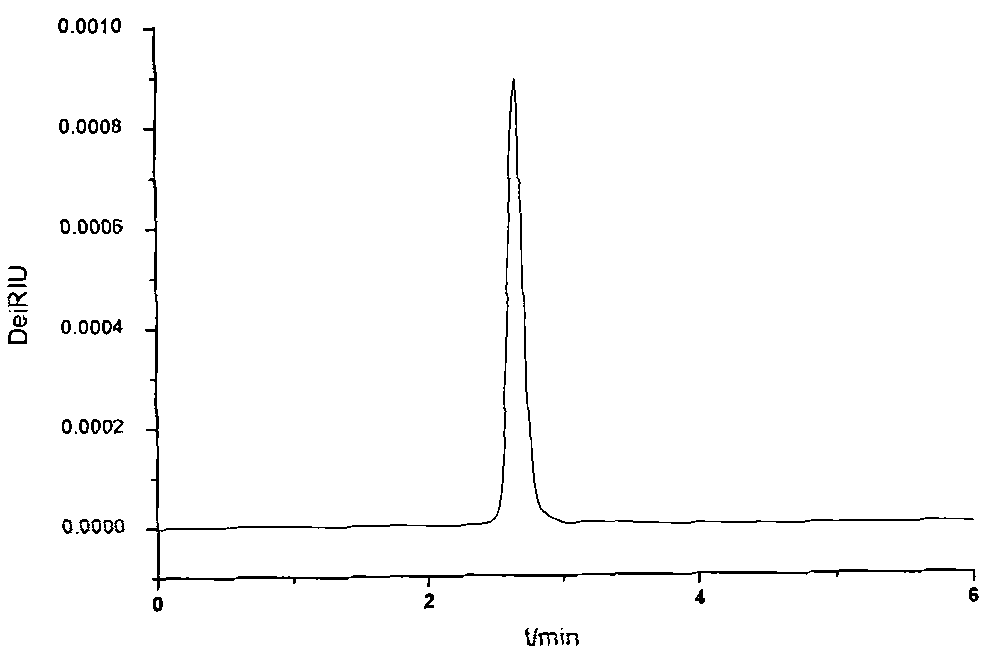
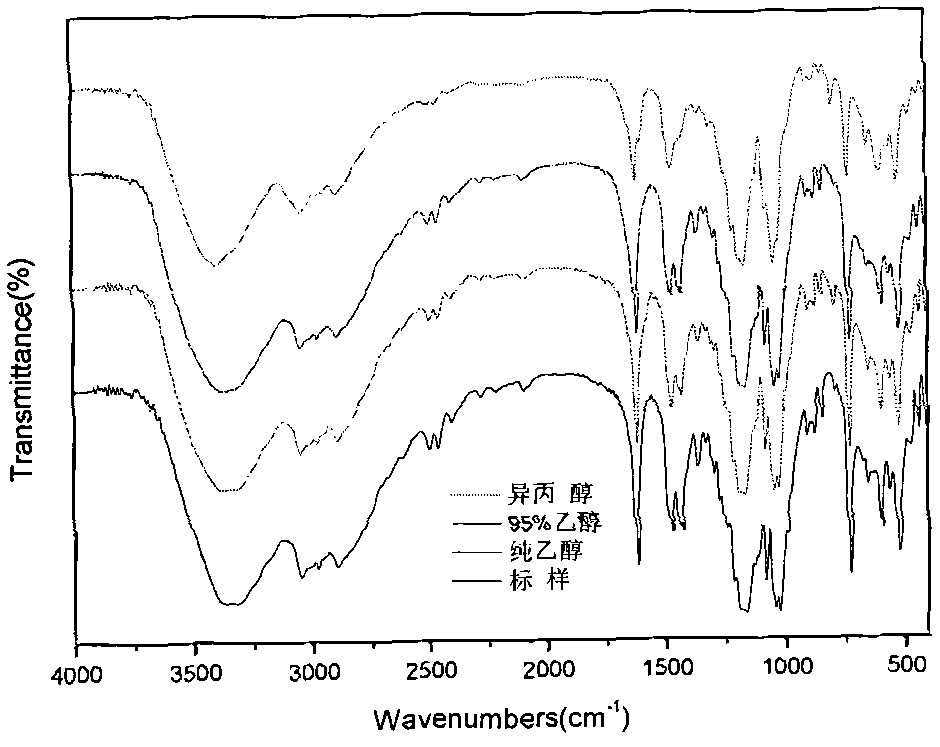
[0019] Put 24.22g (0.2mol) of trishydroxymethylaminomethane and 24.43g (0.2mol) of 1,3-propane sultone into a 500mL four-necked flask with a stirring paddle, a thermometer, and a condenser, and add About 200mL of 95% alcohol is used as a solvent, stirred and dissolved at a speed of 150 rpm, the reaction system is heated to boiling, and the ethanol is condensed and refluxed. After reacting for about 2 hours, cool to room temperature, and then put it in the refrigerator to cool to 0°C or below. After the product is fully crystallized, take it out and filter. The filter cake is the obtained crude product. The crude product was washed 2-3 times with absolute ethanol, put into an oven and dried at 80° C. for 24 hours to obtain the final product in the form of white powder. According to high-performance liquid phase analysis, its purity is 98.09%, its yield is 71.77%, and its melting point is 229.1-229.4°C.
Embodiment 2
[0021] Put 24.22g (0.2mol) of trishydroxymethylaminomethane and 24.43g (0.2mol) of 1,3-propane sultone into a 500mL four-necked flask with a stirring paddle, a thermometer, and a condenser, and add About 200mL of pure ethanol was used as a solvent, stirred and dissolved at a speed of 150 rpm, the reaction system was heated to boiling, and the ethanol was condensed and refluxed. After reacting for about 2 hours, cool to room temperature, and then put it in the refrigerator to cool to 0°C or below. After the product is fully crystallized, take it out and filter. The filter cake is the obtained crude product. The crude product was washed 2-3 times with absolute ethanol, put into an oven and dried at 75°C for 24 hours to obtain 36.26g of the final product in the form of white powder, with a yield of 74.61% and a melting point of 229.7-230.0°C.
Embodiment 3
[0023] Put 24.22g (0.2mol) of trishydroxymethylaminomethane and 24.43g (0.2mol) of 1,3-propane sultone into a 500mL four-necked flask with a stirring paddle, a thermometer, and a condenser, and add About 200mL of n-propanol was used as a solvent, stirred and dissolved at a speed of 150 rpm, the reaction system was heated to boiling, and the n-propanol was condensed and refluxed. After reacting for about 2 hours, cool to room temperature, and then put it in the refrigerator to cool to 0°C or below. After the product is fully crystallized, take it out and filter. The filter cake is the obtained crude product. The crude product was washed 2-3 times with absolute ethanol, put into an oven and dried at 80°C for 24 hours to obtain 36.40 g of a white powdery final product with a yield of 74.82% and a melting point of 229.5-229.9°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com