Preparation method for a series of acridine chemiluminescence agents
A chemiluminescence and acridine technology, applied in the direction of organic chemistry, can solve the problems of high price, complex preparation process, single acridine compound, etc., and achieve the effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] R=H, the preparation of acridine sulfonamide Ⅰ:
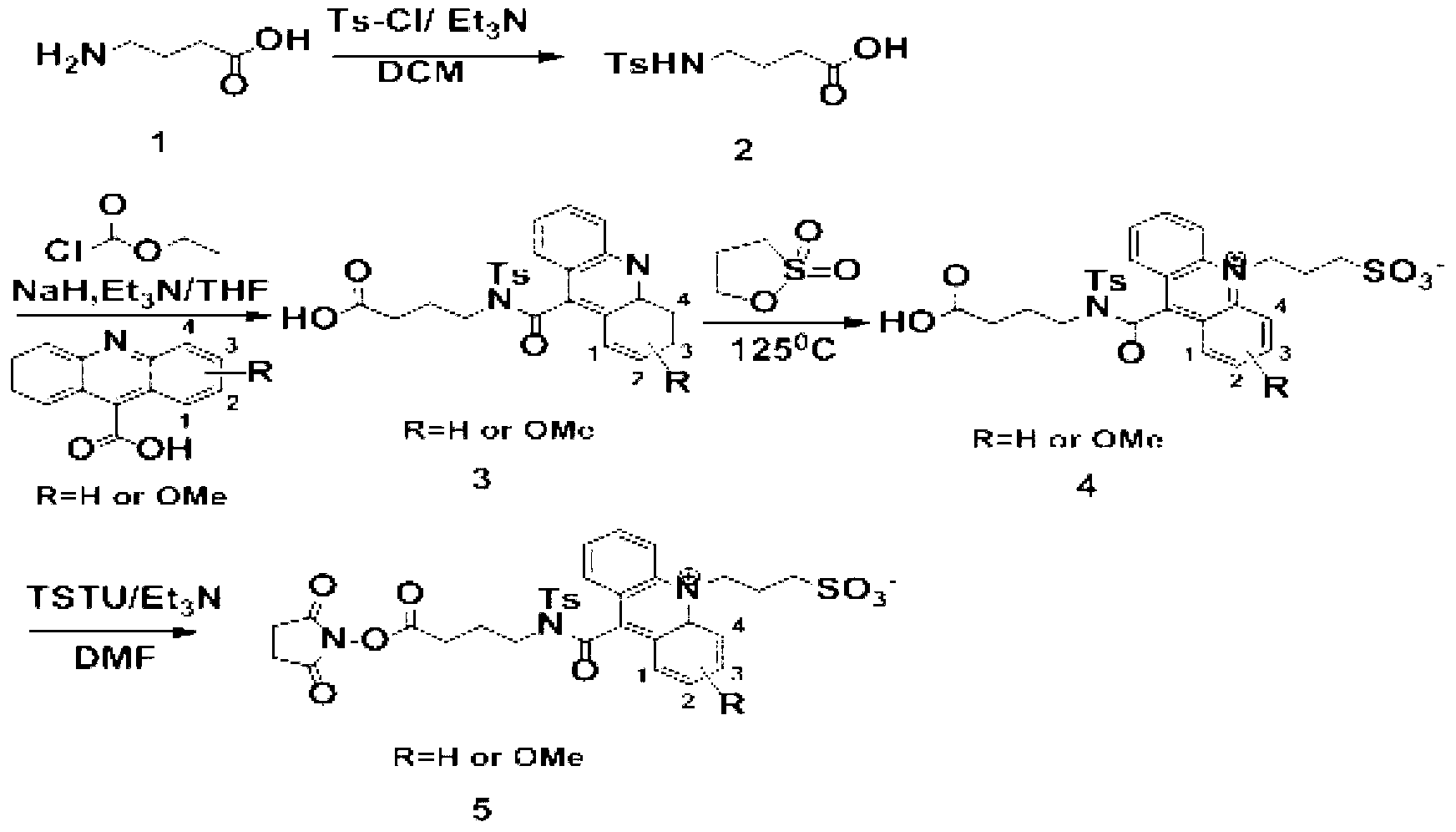
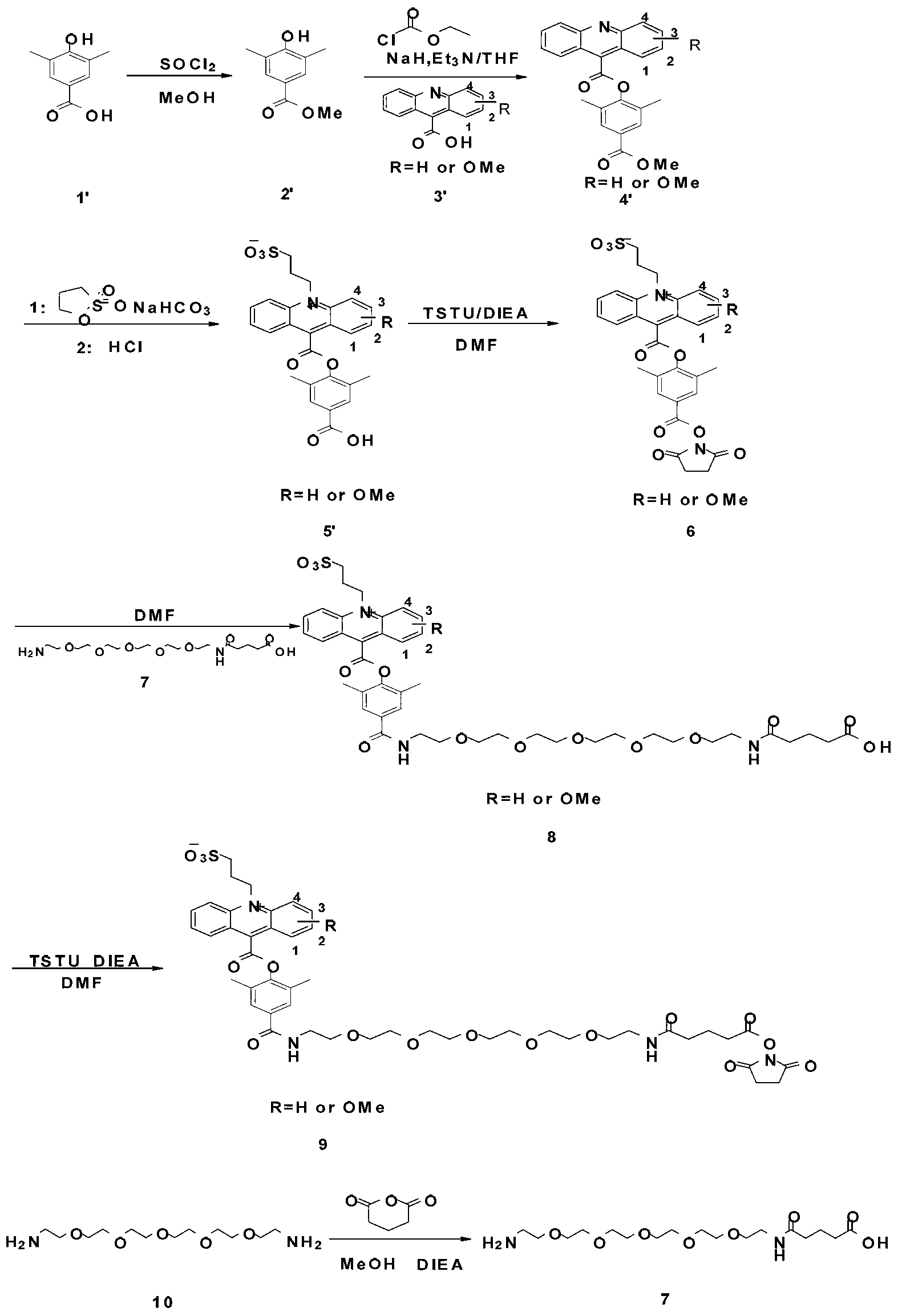
[0033] The first step: preparation of compound 2. Put 1.03g (10mmol) of compound 1, namely 4-aminobutyric acid, in a round bottom burner, add 40mL of dichloromethane, stir magnetically, cool to 0°C, add 2.02g (20mmol) of triethylamine and p-toluenesulfonate in sequence Acyl chloride 2.10g (11mmol), kept at 0°C for 1h, adjusted the pH to 3 with 1mol / L hydrochloric acid after the reaction, then extracted the product twice with 200mL ethyl acetate, took the organic phase and added anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure to obtain 2.19 g (8.50 mmol) of the product compound 2 with a yield of 85%.
[0034] The second step: the preparation of compound 3. Put 1.12g (5mmol) of 9-acridinecarboxylic acid in a round-bottomed flask, add 25mL of tetrahydrofuran to dissolve it, stir magnetically, cool to -10°C, add 0.61g (6mmol) of triethylamine and 0.54% ethyl chloroformate in sequence ...
Embodiment 2
[0039] R=OMe (this substituent is attached as figure 1 On the carbon of the 1 position marked above), the preparation of acridine sulfonamide Ⅱ:
[0040] The first step: preparation of compound 2. Put 1.03g (10mmol) of compound 1, namely 4-aminobutyric acid, in a round bottom burner, add 40mL of dichloromethane, stir magnetically, cool to 0°C, add 2.02g (20mmol) of triethylamine and p-toluenesulfonate in sequence Acyl chloride 2.10g (11mmol), kept at 0°C for 1h, adjusted the pH to 3 with 1mol / L hydrochloric acid after the reaction, then extracted the product twice with 200mL ethyl acetate, took the organic phase and added anhydrous sodium sulfate After drying, the solvent was removed under reduced pressure to obtain 2.19 g (8.50 mmol) of the product compound 2 with a yield of 85%.
[0041] The second step: the preparation of compound 3, put 1.27g (5mmol) of 1-methoxy-9-acridinecarboxylic acid in a round bottom flask, add 25mL tetrahydrofuran to dissolve it, stir magnetically...
Embodiment 3
[0046] R=OMe, (this substituent is attached as figure 1 On the carbon of the 2 position marked above), the preparation of acridine sulfonamide Ⅲ
[0047] The first step: preparation of compound 2. Put 1.03g (10mmol) of compound 1, namely 4-aminobutyric acid, in a round bottom burner, add 40mL of dichloromethane, stir magnetically, cool to 0°C, add 2.02g (20mmol) of triethylamine and p-toluenesulfonate in sequence Acyl chloride 2.10g (11mmol), kept at 0°C for 1h, adjusted the pH to 3 with 1mol / L hydrochloric acid after the reaction, then extracted the product twice with 200mL ethyl acetate, took the organic phase and added anhydrous sodium sulfate Drying, removal of solvent under reduced pressure gives product 2.19g (8.50mmol) of compound 2, and the yield is 85%;
[0048] The second step: the preparation of compound 3. Put 1.27g (5mmol) of 2-methoxy-9-acridinecarboxylic acid in a round-bottomed flask, add 25mL of tetrahydrofuran to dissolve it, stir it magnetically, cool to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com