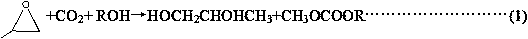Method of one-step synthesis of asymmetric carbonate and co-production of 1,2-propylene glycol by using propylene oxide
A propylene oxide, asymmetric technology, used in the preparation of carbon dioxide or inorganic carbonate, chemical instruments and methods, products, etc., can solve the problem of increased operating costs, low reactant conversion and product selectivity, and high energy consumption. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] 1. Preparation method of mesoporous molecular sieve carrier:
[0065] In the present invention, orderly acid-base treatment is carried out on different carriers, and finally the molecular sieve carrier with the compound pore structure of mesoporous and micropores is prepared by high-temperature roasting. The purpose of the acid treatment is to remove the Al in the molecular sieve framework, so as to realize pore formation. The main function of the alkali treatment is to remove the Si in the molecular sieve framework to make the molecular sieve form a mesoporous structure. The preparation process includes the following steps:
[0066] 1) Dealumination: Add a certain mass of M-S carrier to a certain volume of acidic solution with a concentration of 0.11 mol / L, then stir and reflux in an oil bath at 100°C for 6 h, then filter, wash, and dry at 120°C for 8 h. The dealuminated M-S-DAl carrier is obtained.
[0067] 2) Desiliconization: Add the M-S-DAl carrier prepared in pro...
Embodiment 1
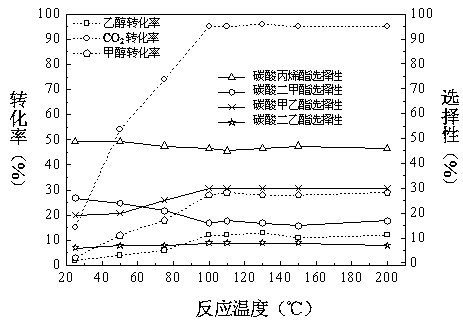
[0101] In the still of 50L slurry bed reactor, reaction pressure 10MPa, reaction raw material propylene oxide: carbon dioxide: methyl alcohol: ethanol=1:1:3:2, drop into each 1.2 kg of the neutral catalyst that above-mentioned preparation method obtains respectively, reaction solution The total volume is 40 L. The reaction was mechanically stirred at a reaction temperature of 100°C. After 10 hours of reaction, samples were taken for chromatographic analysis and calculation. The conversion rate of raw materials and product selectivity are shown in Table 1.
[0102] Table 1 Effects of different types of catalysts on feedstock conversion and product selectivity
[0103]
[0104] When the reaction is catalyzed by a neutral catalyst, the conversion rates of propylene oxide (PO) and carbon dioxide are basically the same, because propylene oxide only reacts with carbon dioxide to form propylene carbonate, and no other side reactions occur. In the following examples, neutral catal...
Embodiment 2
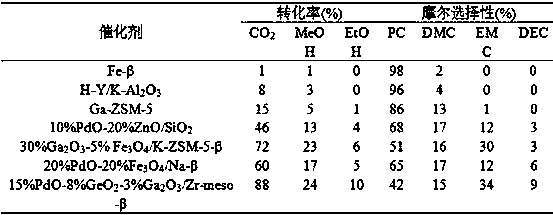
[0106] In a 50L slurry bed reactor, the reaction pressure is 10MPa, the reaction raw materials are propylene oxide: carbon dioxide: methanol: other alcohols = 1:1:3:2, and 15%PdO-8%GeO is added 2 -3%Ga 2 o 3 / Zr-meso-β catalyst 1.2 kg, the total volume of the reaction solution is 40L. The reaction was mechanically stirred at a reaction temperature of 100°C. After 10 hours of reaction, samples were taken for chromatographic analysis and calculation. The conversion rate of raw materials and product selectivity are shown in Table 2.
[0107] Table 2 Effects of different alcohols on feedstock conversion and product selectivity
[0108] It can be seen from Table 2 that when different alcohols are used as reaction raw materials to synthesize asymmetric carbonate esters, the reaction results are quite different. As the R group becomes complex, the conversion rate of ROH gradually decreases, and the selectivity of asymmetric carbonate esters also decreases. Gradually decreases. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com