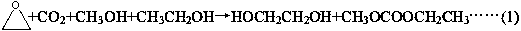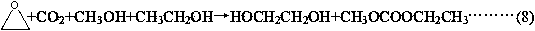Process for one-step synthesis of methyl ethyl carbonate and co-production of ethylene glycol by using ethylene oxide
A technology of ethyl methyl carbonate and ethylene oxide, applied in the field of co-production of ethylene glycol, can solve the problems of increased operating costs, high energy consumption in industrial production processes, low conversion rate of reactants and low selectivity of products, etc. To achieve the effect of short synthesis route and clean reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
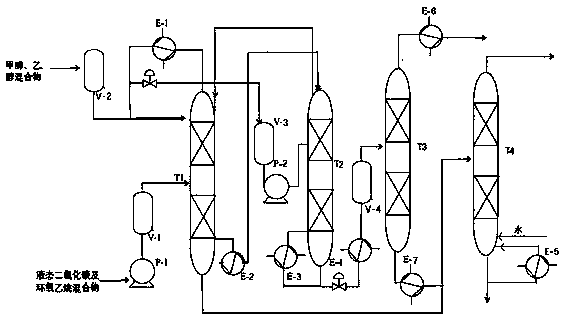
[0065] Using carbon dioxide, ethylene oxide, methanol and ethanol as raw materials, the reaction generates ethyl methyl carbonate, the substance ratio of methanol and ethanol is 3:2, and the excess carbon dioxide and ethylene oxide are recycled.
[0066] The catalyst is basic composite material 15%BaO-5%CaO-3%La 2 O 3 / Cs-meso-EMT, the dosage is 3% of the total mass of the raw materials, and the ratio of the cross-sectional area of the initial distillation section to the side line section is 1:1.
[0067] The operating conditions are as follows:
[0068] The first reactor T1: the diameter of the tower is 1000mm; the height of the tower is 19000mm; the number of plates in the common rectifying section, the initial distillation section, the side line section and the common stripping section are 10, 20, 30 and 15 respectively; the pressure in the tower is 5MPa; The top temperature is 70°C; the column bottom temperature of the common stripping section is 150°C; the column bott...
Embodiment 2
[0077] Under the operating conditions of Example 1, the total raw material mass flow rate was 2500kg / h, and when the rectification pressure was 3MPa, the reflux ratio of the T2 pressurized rectification tower was changed, and the mass fraction of methyl ethyl carbonate at the bottom of the tower was changed as shown in Table 2 .
[0078] Table 2 Influence of different reflux ratios on the change of the mass fraction of methyl ethyl carbonate at the bottom of the T2 pressurized distillation column
[0079]
[0080] It can be seen from Table 2 that with the increase of the reflux ratio, the mass fraction of EMC at the bottom of the T2 pressurized rectification column first increases and then the increase rate obviously slows down. The reason is that with the increase of the reflux ratio, more methanol, ethanol, DMC and the azeotrope of DMC and methanol at the top of the column are taken out of the liquid phase, and the mass fraction of DMC in the column fraction decreases, an...
Embodiment 3
[0082] Under the operating conditions of Example 1, when the total raw material mass flow rate was 2500kg / h, the pressure of the T2 pressurized rectification tower was changed, and the changes in the mass fractions of the components at the bottom of the tower bottom were shown in Table 3.
[0083] Table 3 Effects of different pressures on the composition of the bottom of the T2 distillation column
[0084]
[0085] It can be seen from Table 3 that with the increase of reaction pressure, the mass fraction of methanol and ethanol in each liquid phase composition decreases, because the boiling point difference of the azeotrope composition increases at higher pressure, and the azeotrope is easier to separate. However, when the pressure is higher than 3MPa, the components basically remain unchanged. Since the high pressure requires high equipment, the input cost will be greatly increased, so 3MPa is the best condition for the rectification process.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com