Tech. of producing ortho nitro methyl-phenoxide para nitro methyl-phenoxide and meta nitro chlorobenzene from chlorobenzene
A technology of o-nitroanisole and p-nitroanisole is applied in the field of fine chemical industry, can solve the problems of high cost, large equipment investment and operation cost, and high raw material cost, and achieves low production cost, production stability and high quality. The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
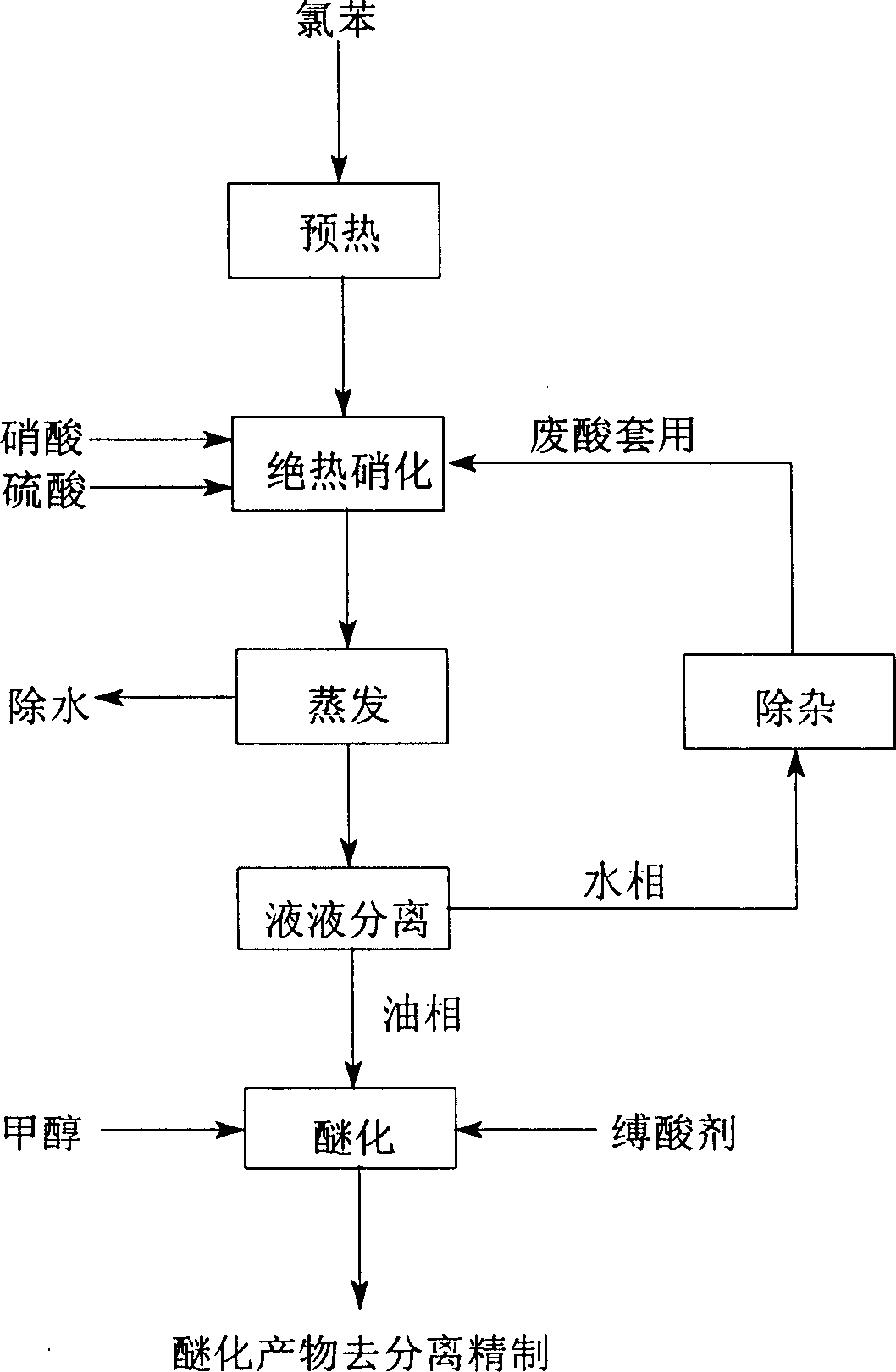
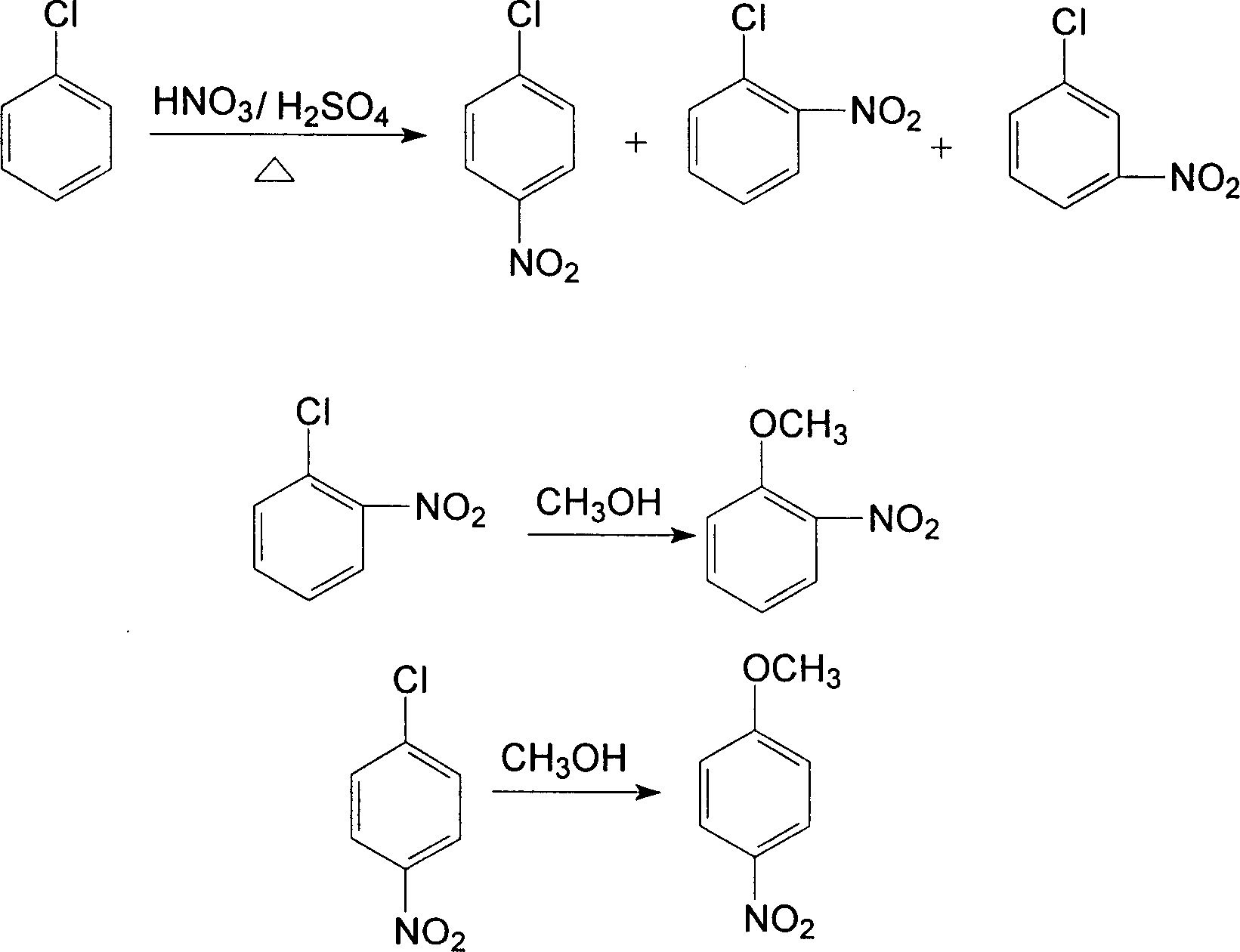
[0021] As shown in the figure, the process of producing o-nitroanisole, p-nitroanisole and m-nitrochlorobenzene with chlorobenzene, the process steps are as follows:
[0022] The first step, in the continuous static mixer adiabatic nitration reactor, the molar ratio of chlorobenzene to nitric acid is 1:1.05, the dehydration value of the mixed acid is between 2.0 and 3.2, and the continuous adiabatic operation occurs between 80 and 120°C Nitration reaction, the chlorobenzene raw material is preheated and then undergoes a continuous nitration reaction with the mixed acid in an adiabatic nitration reactor. The mixed acid in the reaction process is a mixture of industrial grade nitric acid and sulfuric acid;
[0023] In the second step, the decompression of the material through the continuous adiabatic nitration reaction is reduced to 28.0kPa, water and remaining nitric acid are distilled off, and 40% to 75% of waste acid is concentrated into 60% to 90% of sulfuric acid;
[0024] ...
Embodiment 2
[0029] A process for continuous adiabatic nitration used in a jet jet nitration reactor, the process steps are as follows:
[0030] (1). Pre-preparation of mixed acid
[0031] Mix 98% sulfuric acid, 95% nitric acid and 65% nitric acid in a certain proportion;
[0032] (2).Continuous adiabatic nitrification reaction
[0033] In the jet jet nitration reactor, the chlorobenzene raw material was preheated and then reacted continuously with the mixed acid in the adiabatic nitration reactor. The reaction results are shown in Table 1.
[0034] Table 1
[0035] implementation plan
Embodiment 3
[0037] A high-pressure etherification process of chlorobenzene nitration product in a stirred tank reactor, the process steps are as follows:
[0038] (1). After the liquid-liquid separation of the nitration product in Example 2, it is added to the high-pressure reaction stirred tank, and the ratio of methanol and sodium hydroxide is set to 100ml: 1 between 18g and 1-p-nitrochlorobenzene The molar ratio with sodium hydroxide is 1.0:1.05, put into the autoclave, heat up to 70°C in airtight, keep warm for 3 hours; slowly raise the temperature to 80°C, keep warm for 4 hours, then raise the temperature to 90°C, keep warm for 16 hours. Sampling was carried out to detect the content of o- and p-nitroanisole.
[0039] (2). After the etherification reaction product is recovered by evaporation of the etherification agent, the m-nitrochlorobenzene in it is recovered by vacuum evaporation to obtain the crude m-nitrochlorobenzene, which is crystallized in the etherification agent Separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com