A kind of synthetic method of 2-amino-4-acetamidoanisole
A technology of acetamidoanisole and p-aminoanisole, which is applied in the field of synthesis of 2-amino-4-acetamidoanisole, can solve problems such as increased raw material costs, achieve low equipment corrosion, and avoid raw materials being easy to use. Oxidation, high conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
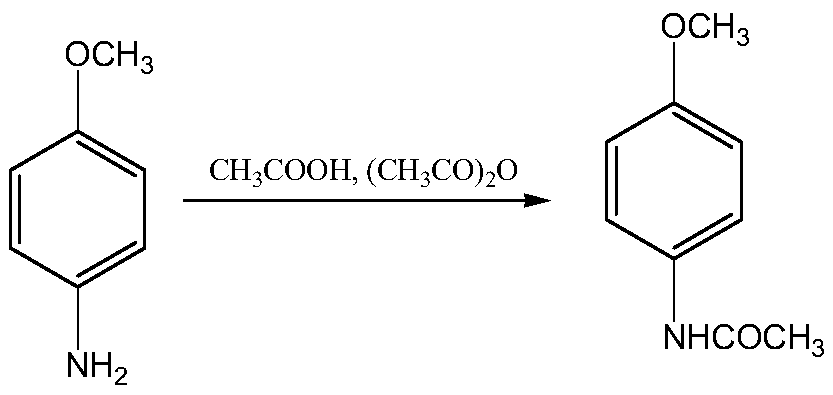
[0062] Embodiment 1, synthetic 2-amino-4-acetamidoanisole
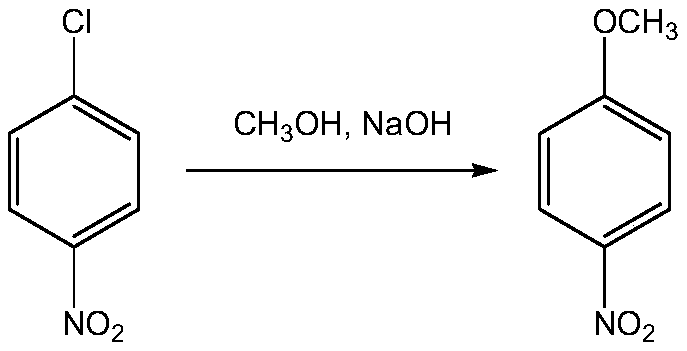
[0063] (1) etherification reaction
[0064] Add 1570kg of p-nitrochlorobenzene and 1920kg of methanol to a 3000L etherification reaction kettle, then raise the reaction temperature to 70°C, gradually add 420kg of sodium hydroxide to it, and carry out the etherification reaction. Heat filtration at 40°C to obtain p-nitroanisole. The yield is 97.2%, and the melting point is 50.5-52.0°C. According to GC analysis, its purity is 99.7%, and the filtrate enters the methanol separation tower, and the methanol is recovered through distillation.
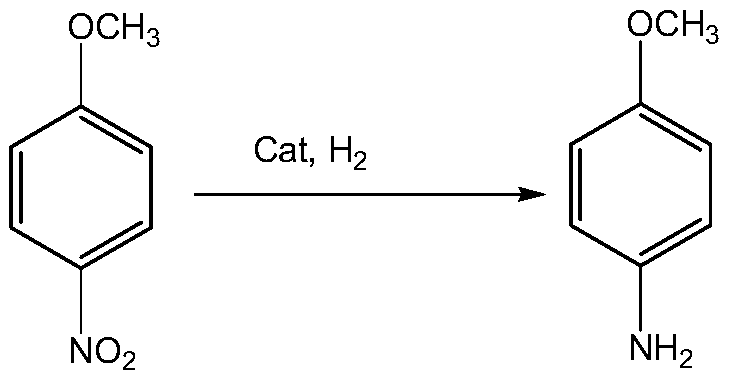
[0065] (2) One catalytic hydrogenation reduction reaction
[0066] With 6000kg p-nitroanisole, 120kg catalyst Ni-Mo-Co-Li (wherein the weight percent of Ni, Mo, Co, Li is 90.0%, 5.0%, 2.0% and 3%, carrier is modified alumina) Add 7500L methanol in the 16000L reactor, feed nitrogen into the reactor, evacuate the air, repeat 5 times, to completely remove the air in the reactor, then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com