Method for preparing p-anisidine through catalytic hydrogenation of p-nitroanisole
A technology of p-nitroanisole and p-aminoanisole, which is applied in the field of p-nitroanisole catalytic hydrogenation to prepare p-aminoanisole, which can solve the complex catalyst preparation process, high production cost, and high equipment requirements. Problems, to achieve non-toxic and non-corrosive separation and reuse, low cost, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
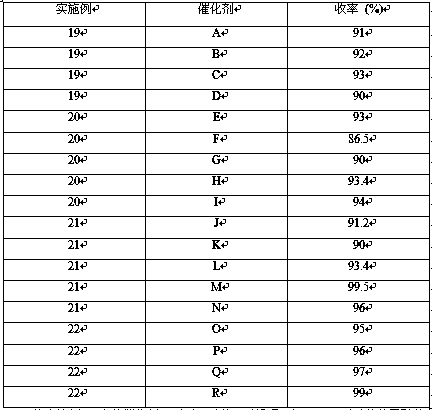
Examples
Embodiment 1
[0019] Embodiment 1 (Ni-Cr / C-SiO 2 )
[0020] Weigh 20 g SiO 2 Add to 150 mL deionized water, stir for 2 h, and disperse evenly; weigh 0.37 g (0.9 mmol) of chromium nitrate nonahydrate, 0.26 g (0.9 mmol) of nickel nitrate hexahydrate, and 2.2 g (20 mmol) of resorcinol, Add 3.25 g (37wt.%) formaldehyde aqueous solution to the above solution, heat and stir in a water bath at 80 °C. Slowly add 1.7 mol / L Na 2 CO 3 50 mL of aqueous solution, stirred for 3 h, centrifuged the mixture, washed to neutral, moved to a drying oven for 4 h at 100 °C, and then dried under N 2 Calcination was carried out at 450 °C for 4 h under protective conditions, and finally the temperature was raised to 450 °C using a temperature-programmed reduction furnace at a hydrogen flow rate of 20 mL / min and a heating rate of 10 °C / min, and then lowered to room temperature after holding for 2 h to obtain catalyst A.
Embodiment 2
[0021] Embodiment 2 (Ni-Fe / C-Al 2 o 3 )
[0022] Weigh 20 g Al 2 o 3 Add to 150 mL deionized water, stir for 2 h, and disperse evenly; weigh 0.6 g (2 mmol) of nickel nitrate hexahydrate, 0.81 g (2 mmol) of iron nitrate nonahydrate, and 0.7 g (6.5 mmol) of resorcinol, 1.1 g (37 wt.%) formaldehyde aqueous solution was added to the above solution, heated and stirred in a water bath at 80 °C. Slowly add 1.7 mol / L Na 2 CO 3 50 mL of aqueous solution, stirred for 3 h, centrifuged the mixture, washed to neutral, moved to a drying oven for 4 h at 100 °C, and then dried under N 2 Calcination was carried out at 450 °C for 4 h under protective conditions, and finally the temperature was raised to 450 °C using a temperature-programmed reduction furnace at a hydrogen flow rate of 20 mL / min and a heating rate of 10 °C / min.
Embodiment 3
[0023] Embodiment 3 (Ni-Zn / C-Al 2 o 3 )
[0024] Weigh 20 g Al 2 o 3 Add to 150 mL deionized water, stir for 2 h, and disperse evenly; weigh 0.3 g (1 mmol) of nickel nitrate hexahydrate, 0.3 g (1 mmol) of zinc nitrate hexahydrate, and 2.2 g (20 mmol) of resorcinol, 3.25 g (37 wt.%) formaldehyde aqueous solution was added to the above solution, heated and stirred in a water bath at 80 °C. Slowly add 1.7 mol / L Na 2 CO 3 50 mL of aqueous solution, stirred for 3 h, centrifuged the mixture, washed to neutral, moved to a drying oven for 4 h at 100 °C, and then dried under N 2 Calcination was carried out at 450 °C for 4 h under protected conditions, and finally the temperature was raised to 450 °C using a temperature-programmed reduction furnace at a hydrogen flow rate of 20 mL / min and a heating rate of 10 °C / min, and then lowered to room temperature after holding for 2 h to obtain catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com