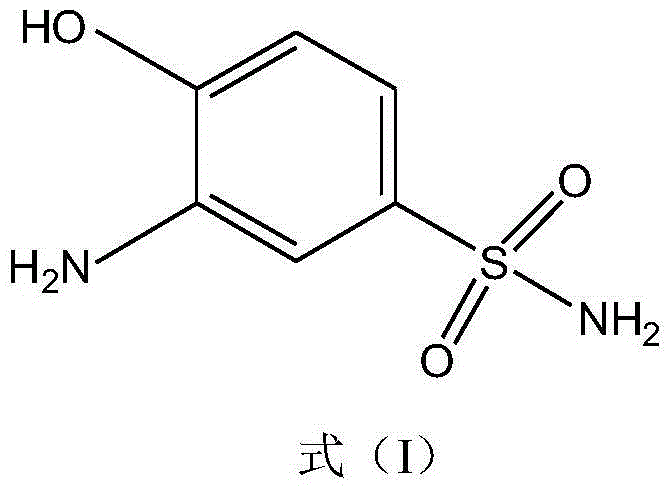
Synthetic method of 2-aminophenol-4-sulfonamide
A synthesis method and technology of aminophenol, applied in the preparation of sulfonic acid amide, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of large amount of chlorosulfonic acid, waste, and low yield, so as to reduce waste and reduce Dosage, the effect of increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthesis of embodiment 1,2-aminophenol-4-sulfonamide
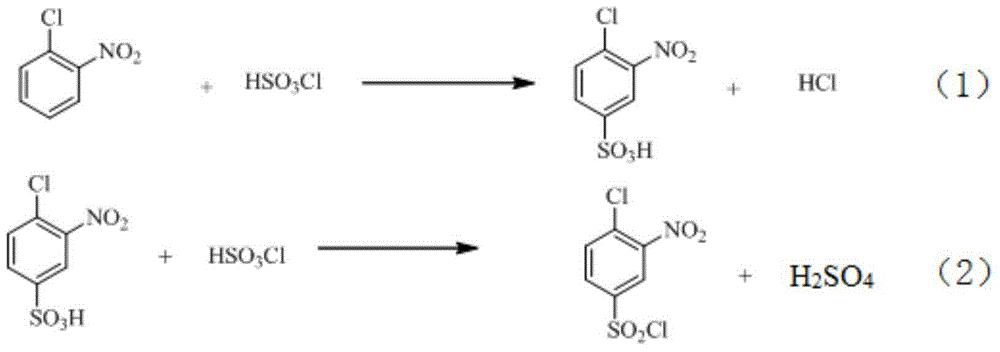
[0041] (1) Chlorosulfonation reaction
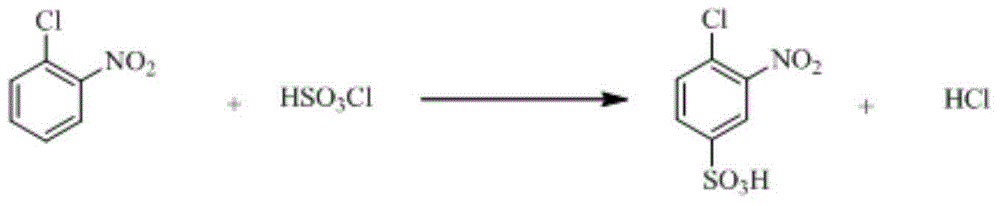
[0042] a) Add 109g of chlorosulfonic acid into the reaction kettle, add 50g of o-nitrochlorobenzene within 1.5 hours, keep warm at 110°C after the addition, and obtain 4-chloro-3-nitrobenzenesulfonic acid through sulfonation reaction for 4 hours solution;
[0043] b) Cool down the solution obtained in step a) to 70° C., add 49.2 g of thionyl chloride and undergo chlorination for 2 hours to obtain 4-chloro-3-nitrobenzenesulfonyl chloride; the product yield is 96.88%.
[0044] (2) Amination reaction
[0045] Dissolve 79.3 g of 4-chloro-3-nitrobenzenesulfonyl chloride obtained in step b) in 120 mL of water, add 120 ml of concentrated ammonia water (16%) at 19°C within 4 hours, then raise the temperature to 36°C, and react for 3.5 hours to obtain 2-Nitrochlorobenzene-4-sulfonamide. The product yield is 90%.
[0046] (3) Hydrolysis and acidification reaction
[0047]Rais...
Embodiment 2
[0050] The synthesis of embodiment 2,2-aminophenol-4-sulfonamide
[0051] (1) Chlorosulfonation reaction
[0052] a) Add chlorosulfonic acid to the reaction kettle, add o-nitrochlorobenzene within 1 hour, keep warm at 100°C after the addition, and obtain 4-chloro-3-nitrobenzenesulfonic acid solution through sulfonation reaction for 3 hours; Wherein the mol ratio of chlorosulfonic acid and o-nitrochlorobenzene is 3:1;
[0053] b) Cool the solution obtained in step a) to 60° C., add thionyl chloride and undergo a chlorination reaction for 1.5 hours to obtain 4-chloro-3-nitrobenzenesulfonyl chloride; the product yield is 96.87%; wherein thionyl chloride and The molar ratio of o-nitrochlorobenzene is 1.3:1.
[0054] (2) Amination reaction
[0055] Dissolve the 4-chloro-3-nitrobenzenesulfonyl chloride obtained in step b) in 120 mL of water, add 120 ml of concentrated ammonia water (16%) at 16°C within 4 hours, then raise the temperature to 37°C, and react for 4.5 hours to obtain...
Embodiment 3
[0060] The synthesis of embodiment 3,2-aminophenol-4-sulfonamide
[0061] (1) Chlorosulfonation reaction
[0062] a) Add chlorosulfonic acid to the reaction kettle, add o-nitrochlorobenzene within 5 hours, keep warm at 120°C after the addition, and obtain 4-chloro-3-nitrobenzenesulfonic acid solution through sulfonation reaction for 5 hours; Wherein the mol ratio of chlorosulfonic acid and o-nitrochlorobenzene is 2.5:1;
[0063] b) Cool the solution obtained in step a) to 65°C, add thionyl chloride and undergo a chlorination reaction for 3.5 hours to obtain 4-chloro-3-nitrobenzenesulfonyl chloride; the product yield is 96.82%; wherein thionyl chloride and The molar ratio of o-nitrochlorobenzene is 1.2:1.
[0064] (2) Amination reaction
[0065] Dissolve the 4-chloro-3-nitrobenzenesulfonyl chloride obtained in step b) in 120 mL of water, add 120 ml of concentrated ammonia water (16%) at 18°C within 4 hours, then raise the temperature to 36°C, and react for 4 hours to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com