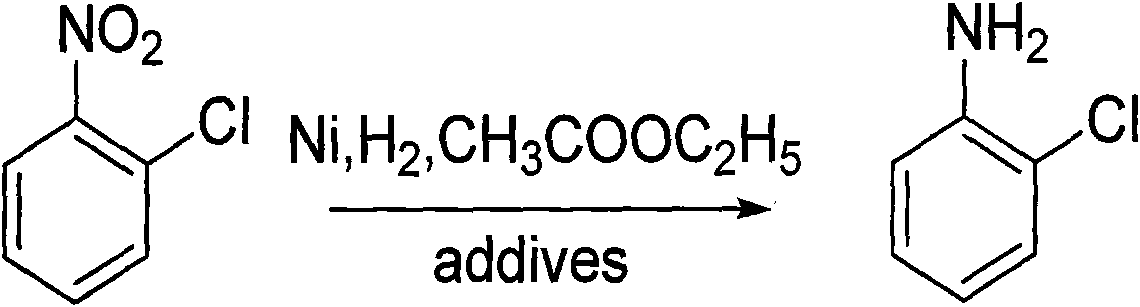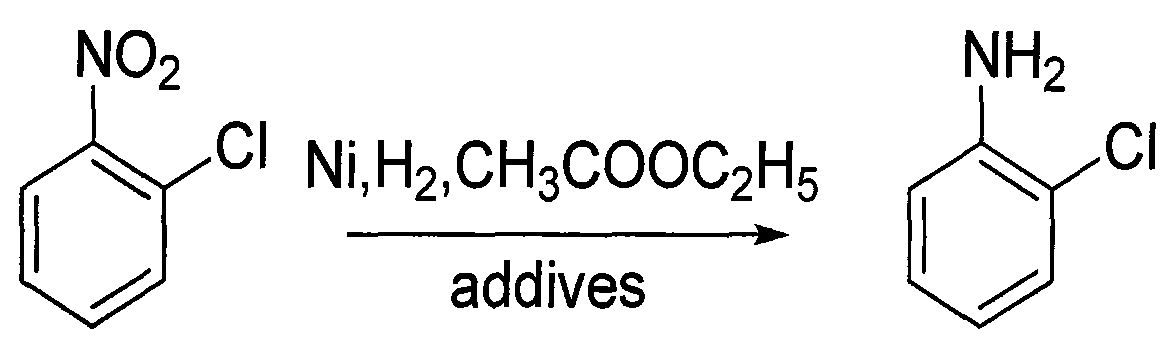Method for preparing o-chloroaniline by catalytic hydrogenation
A technology for catalytic hydrogenation and o-chloroaniline, which is applied in the preparation of aniline and o-chloroaniline, can solve the problems of high energy consumption, complex reduction route, and additional filtration in the electrochemical reduction method, and achieve large-scale Effects of industrialized production, reduced post-treatment process, and reduction of coupling compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment a
[0017] Prior art: 150g of o-nitrochlorobenzene and 300ml of methanol are added to the autoclave, and 7.5g of Raney-Ni catalyst (Ni / Al) and 1.50g of triethylamine are added simultaneously, the autoclave is closed, the hydrogen valve is opened, and the Pass hydrogen into the kettle, ventilate 3 times, adjust the pressure of the kettle to 1.0MPa, stir and heat up to 55-65°C to react, so that the pressure does not change (about 3h), take a sample, and the result of gas chromatography analysis shows that the content of o-chloroaniline is 97.50% . The obtained main product o-chloroaniline was mixed with impurities, and after the catalyst and some insolubles were filtered out, the solvent was recovered and the water was distilled off to obtain 102 g of refined o-chloroaniline (yield 85%, GC99.9%). The residue is about 20.0g, and the residue contains 1-3 / 100 of the coupling compound, which is attached to the rectification tower and is difficult to clean when the rectification tower is...
Embodiment b
[0019] The technology of the present invention: 150g of o-nitrochlorobenzene, 300ml of ethyl acetate are added in the autoclave, 7.5g of Raney-Ni catalyst (Ni / C) is added simultaneously, 1.50g of triethylamine, the airtight autoclave is opened, the hydrogen valve is opened, Introduce hydrogen into the autoclave, ventilate 3 times, adjust the pressure of the autoclave to 1.0MPa, stir and raise the temperature to 55-65°C to react, so that the pressure does not change (about 3h), take a sample, analyze the results by gas chromatography, the content of o-chloroaniline 98.50%. The ethyl acetate phase was obtained by liquid separation, the solvent was recovered and rectified to obtain 110 g of refined o-chloroaniline (yield 90%, GC99.9%). The residue is about 10.0g, and due to the use of ethyl acetate solvent and neutral catalyst, there is no need to filter, just a simple liquid separation, and the coupling compound in the reaction is only 1 / 2 or even less than that of the prior art...
Embodiment 1
[0021] Add 50 g of o-nitrochlorobenzene and 200 ml of ethyl acetate into the autoclave, add 2.5 g of Raney-Ni catalyst (Ni / C) and 1.0 g of dicyandiamide at the same time, close the autoclave, open the hydrogen valve, and inject Pass in hydrogen, ventilate 3 times, adjust the pressure of the kettle to 1.0MPa, stir and heat up to 55-65°C to react (replenish hydrogen repeatedly), so that the pressure does not change, take a sample, and the result of gas chromatography analysis shows that the content of o-chloroaniline is 97.80%. The ethyl acetate phase was obtained by liquid separation, and after the solvent was recovered, rectification obtained 36.5 g of refined o-chloroaniline and impurities (yield 90%, GC99.9%). Impurities remain in the kettle, and after testing, the coupling compound in the impurities is less than 1 / 100.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com