Preparation method of edoxaban
An edoxaban and compound technology, applied in the field of medicinal chemistry, can solve the problems of hidden dangers of production safety, easy to catch fire, expensive, etc., and achieve the effects of good product quality, stable yield and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
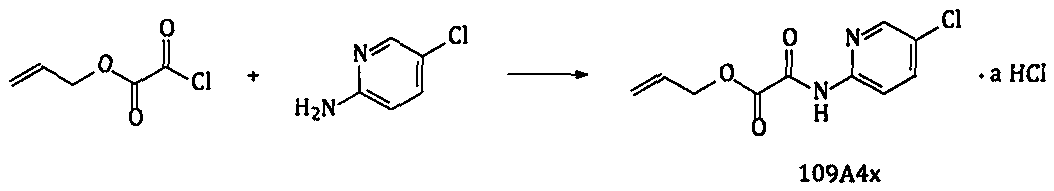
[0073]Example 1 Synthesis of 2-[(5-chloro-2-pyridine)amino]-2-oxoacetate allyl hydrochloride
[0074]
[0075] Add 1000g of acetonitrile to the reaction flask, add 200g (1.556mol) of 2-amino-5-chloropyridine, heat to 30-35°C, add 255g (1.717mol) of monoallyl oxalyl chloride dropwise, and keep the temperature constant during the dropwise addition process. Over 50°C; after the dropwise addition, keep warm at 45-50°C for 3-4 hours. After the reaction is completed, lower the temperature to 0-5°C and keep warm for about 2-4 hours. Filter to collect the solid; add the obtained wet product to 1500g of water, beat at room temperature for 2 to 3 hours; filter, rinse with water, collect the solid, and dry to obtain about 416g of the dry product of 109A4-10 (theoretical amount: 431.1g). Yield 96.5%.
Embodiment 2
[0076] Example 2 Synthesis of allyl 2-[(5-chloro-2-pyridine)amino]-2-oxoacetate
[0077]
[0078] Add 900g ethyl acetate to the reaction flask; add 100g (360.9mmol) 109A4-10; under stirring, add 40g (395.3mmol) triethylamine, heat to 40-45°C, keep stirring for about 3-5hr; filter while hot , and the filtrate was collected; ethyl acetate was concentrated under reduced pressure to obtain a residue of about 95 g. Add about 50g of ethyl acetate to the residue, stir to disperse evenly, then add 300g of petroleum ether, heat to 40-45°C, stir for about 1hr; cool to 0-5°C and keep warm for about 1-2hr. The solid was collected by filtration and dried to obtain about 77 g of dry product of 109A4-00 (theoretical amount: 86.8 g). Yield: 88.7%.
Embodiment 3
[0079] Example 3 Synthesis of allyl 2-[(5-chloro-2-pyridine)amino]-2-oxoacetate
[0080]
[0081] Add 1500g ethyl acetate and 300g water to the reaction flask; add 150g (541.3mmol) 109A4-10; add dropwise a solution prepared by 54g (642.8mmol) sodium bicarbonate and 600g water, adjust to pH=8; keep warm at 20 Stir and extract at ~25°C; separate the liquids, and then extract the aqueous phase with about 750 g of ethyl acetate; combine the organic phases and wash once with saturated saline solution; add anhydrous sodium sulfate to dry; filter and collect the filtrate; concentrate the ethyl acetate under reduced pressure, A residue of about 136 g was obtained. Add about 260g of ethyl acetate to the residue, stir to disperse evenly, then add 400g of petroleum ether, heat to 40-45°C, stir for about 1hr; cool to 0-5°C and keep warm for about 1-2hr. The solid was collected by filtration and dried to obtain about 124 g of dry product of 109A4-00 (theoretical amount: 130.3 g). Yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com