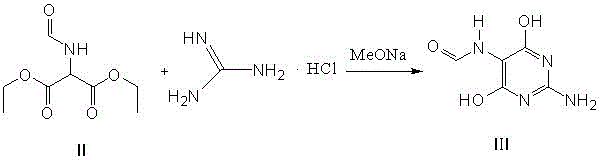
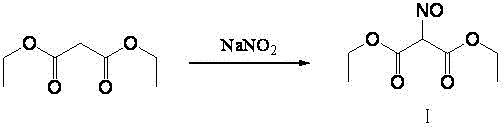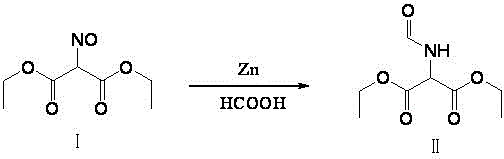Preparation method of 2-amino-4,6-dichloro-5-carboxamidopyrimidine
A technology of formamide and pyrimidine, applied in the field of chemical pharmacy, can solve the problems of complex operation, difficult process control, by-products, etc., and achieve the effects of improving reaction yield, eliminating by-products, and simplifying operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of diethyl isonitrosomalonate (I):
[0035] Add acetic acid: 92ml, diethyl malonate: 80g into a 500ml four-necked bottle, stir and cool to below 10 degrees, slowly add 40% sodium nitrite aqueous solution: 250g, keep the temperature at 5-10 degrees during the dropping After the dropwise addition, the temperature was slowly raised to 40-45° C. for 3.5 hours. After the reaction was completed, it was cooled to 10° C., allowed to stand for stratification, and the lower water layer was removed to obtain the upper oil layer as crude product, weighing 92.6 g, yield: 98%.
Embodiment 2
[0037] Synthesis of diethyl isonitrosomalonate (I):
[0038]Add acetic acid: 150ml, diethyl malonate: 80g into a 500ml four-necked bottle, stir and cool to below 10 degrees, slowly add 40% sodium nitrite aqueous solution: 300g, keep the temperature at 5-10 degrees during the dropping After the dropwise addition is completed, slowly raise the temperature to 30-35°C and keep it warm for 5 hours. After the reaction is completed, cool to 10°C, let it stand for stratification, remove the lower water layer, and obtain the upper oil layer which is diethyl isonitrosomalonate Ⅰ. Crude product, weighing 90.4g, yield: 95%.
Embodiment 3
[0040] Synthesis of diethyl formylaminomalonate (Ⅱ):
[0041] Add crude diethyl isonitrosomalonate I: 92.6g, formic acid: 260g into a 1000ml four-necked bottle, add 90g of zinc powder in batches under stirring, during the addition, control the temperature at 30-32°C, and raise the temperature after the addition After 6 hours of reflux reaction, after the reaction is over, cool down to room temperature, add 200 g of water to recover formic acid under reduced pressure, a large amount of solids precipitate out after recovery, cool down to 10 degrees and filter to obtain a white solid, and dry to obtain the product formylaminomalonic acid di Ethyl ester II: 93.8 g, yield: 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com