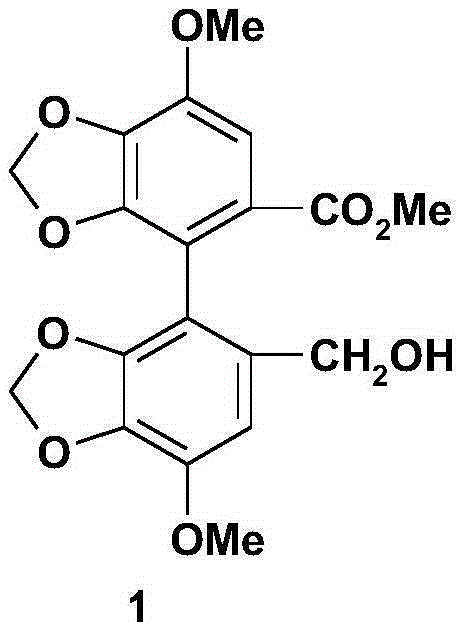
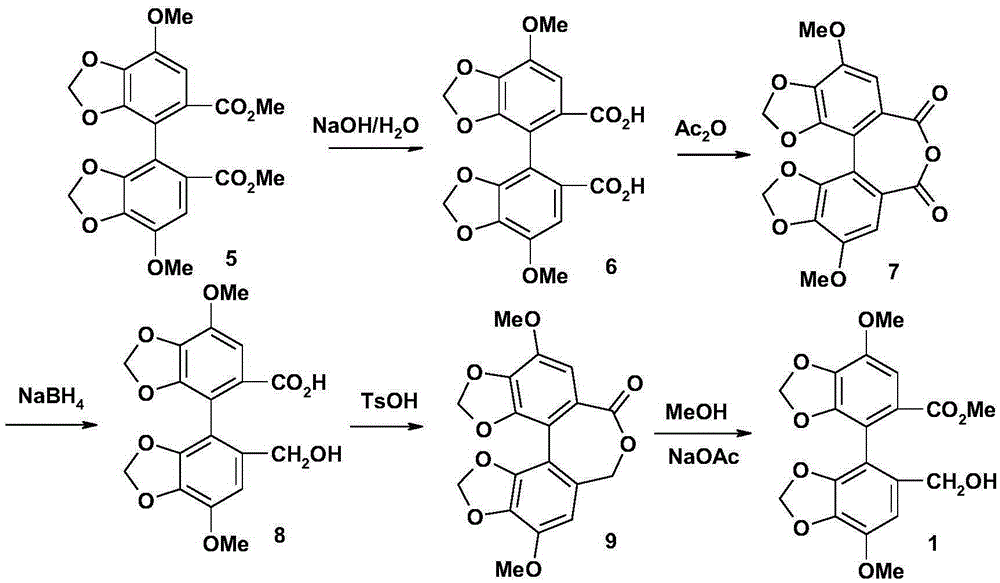
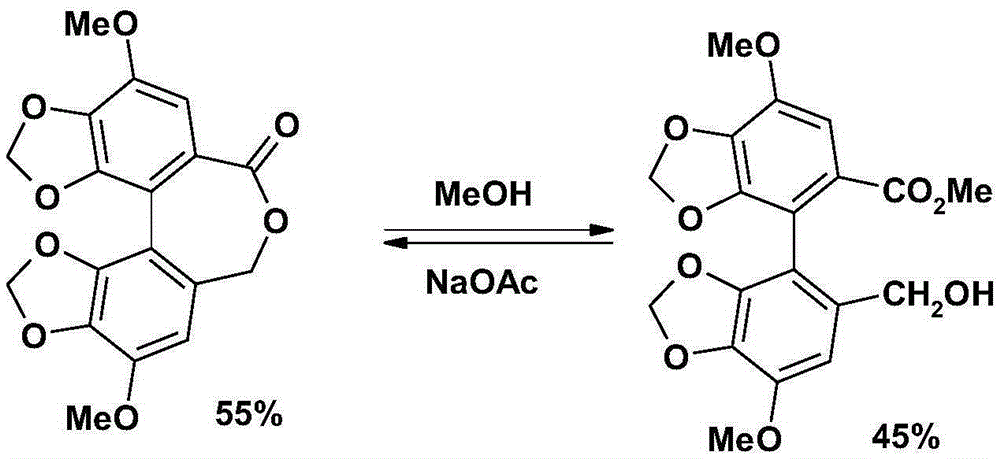
Bicyclol preparation method and intermediate compound thereof
A compound and mixture technology, applied in the field of organic compound synthesis, can solve the problems of low total yield and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: 7,7'-dimethoxy 5'-(2-thiothiazoline-3-carbonyl)-[4,4']bis[benzo[1,3]dioxolane ]-5-formic acid methyl ester (formula (4) compound, wherein R 1 , R 2 , R 3 Respectively is the preparation of methyl)
[0091] (1) 5'-chlorocarbonyl-7,7'-dimethoxy-[4,4']bis[benzo[1,3]dioxolane]-5-methyl carboxylate (formula ( 3) compound, wherein R 1 , R 2 , R 3 respectively methyl)
[0092]
[0093] 2.0 grams (5 mmol) of monomethyl biphenyl diacid (compound 2) was dissolved in 20 ml of dichloromethane, 1.5 ml (20.8 mmol) of thionyl chloride and 1 drop of dimethylformamide were added, heated to reflux for 2 hours, evaporated After drying, 2.15 g of the title product was obtained, which was directly used in the next reaction.
[0094] (2) 7,7'-dimethoxy 5'-(2-thiothiazoline-3-carbonyl)-[4,4']bis[benzo[1,3]dioxolane] -Methyl 5-formate (compound of formula (4), wherein R 1 , R 2 , R 3 Respectively is the preparation of methyl)
[0095]
[0096] Dissolve 2.1g (~5mmo...
Embodiment 2
[0098] Example 2: 4,4'-dimethoxy-5,6,5',6'-bis(methylenedioxy)-2-hydroxymethyl-2'-methoxycarbonylbiphenyl (formula ( 1) compound, wherein R 1 , R 2 , R 3 are respectively the preparation of methyl);
[0099]
[0100] Dissolve 500 mg (1 mmol) of compound 4 in 5 ml of tetrahydrofuran, cool in an ice bath, and add dropwise a solution of 95 mg (2.5 mmol) of sodium borohydride and 0.2 ml of water in 5 ml of tetrahydrofuran under stirring at a controlled temperature of 0-5°C. After the addition, keep warm at 0-5°C and stir for 30 minutes, then raise the temperature to 30-35°C and stir for 30 minutes. The reaction solution turns from yellow to colorless, and TLC shows that the raw material spots disappear. Then dilute hydrochloric acid was added to remove excess sodium borohydride, tetrahydrofuran was evaporated under reduced pressure, the residue was dissolved in dichloromethane, followed by 5% Na 2 CO 3 Wash, wash with water, anhydrous Na 2 SO 4 dry. After filtration, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com