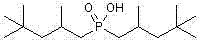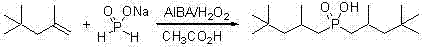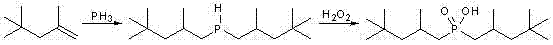Method for synthesizing bis(2,4,4-trimethylpentyl) phosphinic acid with double initiators
The technology of a trimethylpentyl group and a synthesis method is applied in the field of synthesizing bisphosphinic acid, which can solve the problem of high requirements on instruments and equipment, and achieve the effects of short reaction time, simple post-processing method, and non-toxic decomposition products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0023] Add 25.0g (0.28mol) sodium hypophosphite, 25.0g acetic acid and 209.5g (containing α-olefin 1.40mol) diisobutene (α-olefin content 75%), add 37.9g (0.14mol) of azobisisobutylamidine hydrochloride and 15.9g (0.14mol) of 30% hydrogen peroxide, and heat to 60°C under vigorous stirring. After constant temperature reaction for 3 h, it was lowered to room temperature, allowed to stand for stratification, and the aqueous lower layer solution was separated. Wash the upper layer solution with water, add 100mL deionized water and wash 3 times. The β-olefin and a small amount of unreacted α-olefin were distilled off under reduced pressure to obtain 63.6 g of bis(2,4,4-trimethylpentyl)phosphinic acid with a yield of 78.2%. through 31 P-NMR analysis product is composed of:
[0024] Target product bis(2,4,4-trimethylpentyl)phosphinic acid 81.6%
[0025] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 12.2%.
example 2
[0027] By the method of example 1, temperature of reaction is changed into 80 ℃, other methods are unchanged. Bis(2,4,4-trimethylpentyl)phosphinic acid 71.8g, yield 88.3%. through 31 P-NMR analysis product is composed of:
[0028] Target product bis(2,4,4-trimethylpentyl)phosphinic acid 91.3%
[0029] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 5.9%.
example 3
[0031] By the method of example 1, temperature of reaction is changed into 100 ℃, other methods are unchanged. Bis(2,4,4-trimethylpentyl)phosphinic acid 67.6g, yield 83.1%. through 31 P-NMR analysis product is composed of:
[0032] Target product bis(2,4,4-trimethylpentyl)phosphinic acid 86.2%
[0033] Mono-substituted 2,4,4-trimethylpentylphosphonous acid 7.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com