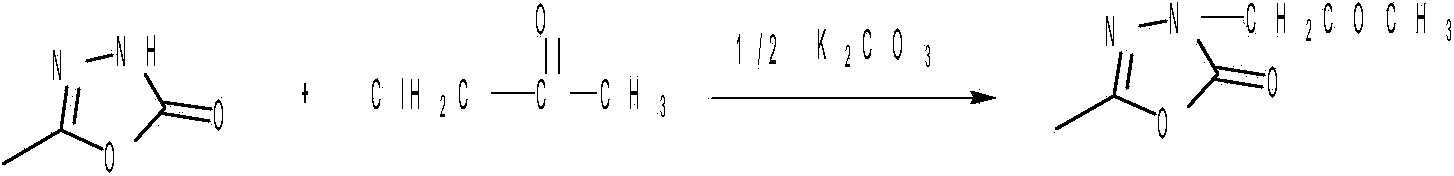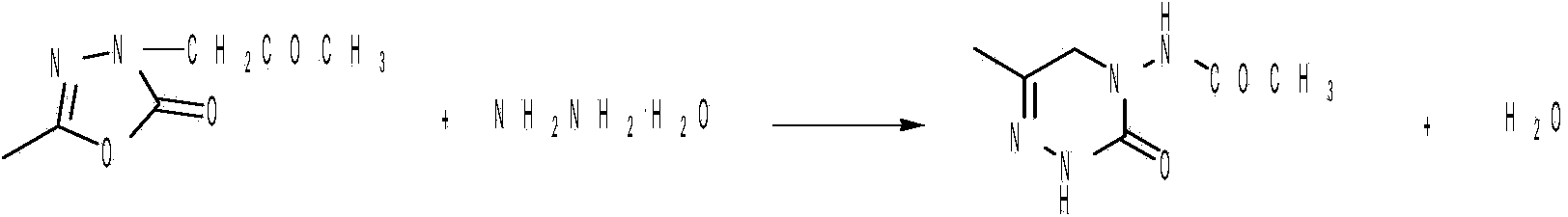High-efficiency and green method for preparing pymetrozine
A pymetrozine and green technology is applied in the field of high-efficiency and green pymetrozine preparation, which can solve the problems such as insufficient utilization of by-products, inability to recycle the solvent, influence on water yield, etc. The effect of reducing the overall production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of embodiment 1 pymetrozine.
[0031] (1) Preparation of acetylhydrazide: Put 1000g of methyl acetate (solvent) into a 3000ml three-necked bottle, and slowly add 500g of hydrazine hydrate dropwise to the three-necked bottle under stirring conditions, and control the dropping time to 2h. The temperature is 40-45°C. After the dropwise addition, the temperature was raised to 75° C. for 4 h. After cooling to room temperature, 725 g of solid acetylhydrazide was obtained after concentration and crystallization (cooling to below 0°C), yield: 98%.
[0032] (2) Preparation of oxadiazolone: put 2000ml of methyl acetate into a 5000ml three-neck flask, add 740g of acetylhydrazide under stirring conditions, and when the temperature is cooled below 0°C, put in 1680g of sodium bicarbonate, and seal the reaction bottle. Continuously feed phosgene into the reaction bottle, the phosgene velocity is 15m 3 / h. After the reaction was completed, the solvent was distille...
Embodiment 2
[0036] The preparation of embodiment 2 acetylhydrazine.
[0037] Put 1,000 g of methyl acetate (solvent) into a 3,000 ml three-necked flask, and slowly add 500 g of hydrazine hydrate dropwise into the there-necked flask under stirring conditions, controlling the dropping time to 2 hours, and controlling the temperature of the reaction solution during the dropping to 40-45°C. After the dropwise addition, the temperature was raised to 60° C. for 5 h. Cool to room temperature, concentrate and crystallize at 0-5°C to obtain 706 g of solid acetylhydrazide, yield: 95%.
Embodiment 3
[0038] The preparation of embodiment 3 acetylhydrazine.
[0039] Put 1,000 g of methyl acetate (solvent) into a 3,000 ml three-necked flask, and slowly add 500 g of hydrazine hydrate dropwise into the there-necked flask under stirring conditions, controlling the dropping time to 2 hours, and controlling the temperature of the reaction solution during the dropping to 40-45°C. After the dropwise addition, the temperature was raised to 85° C. for 5 h. Cool to room temperature, concentrate and crystallize at 0-5°C to obtain 697 g of solid acetylhydrazide, yield: 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com