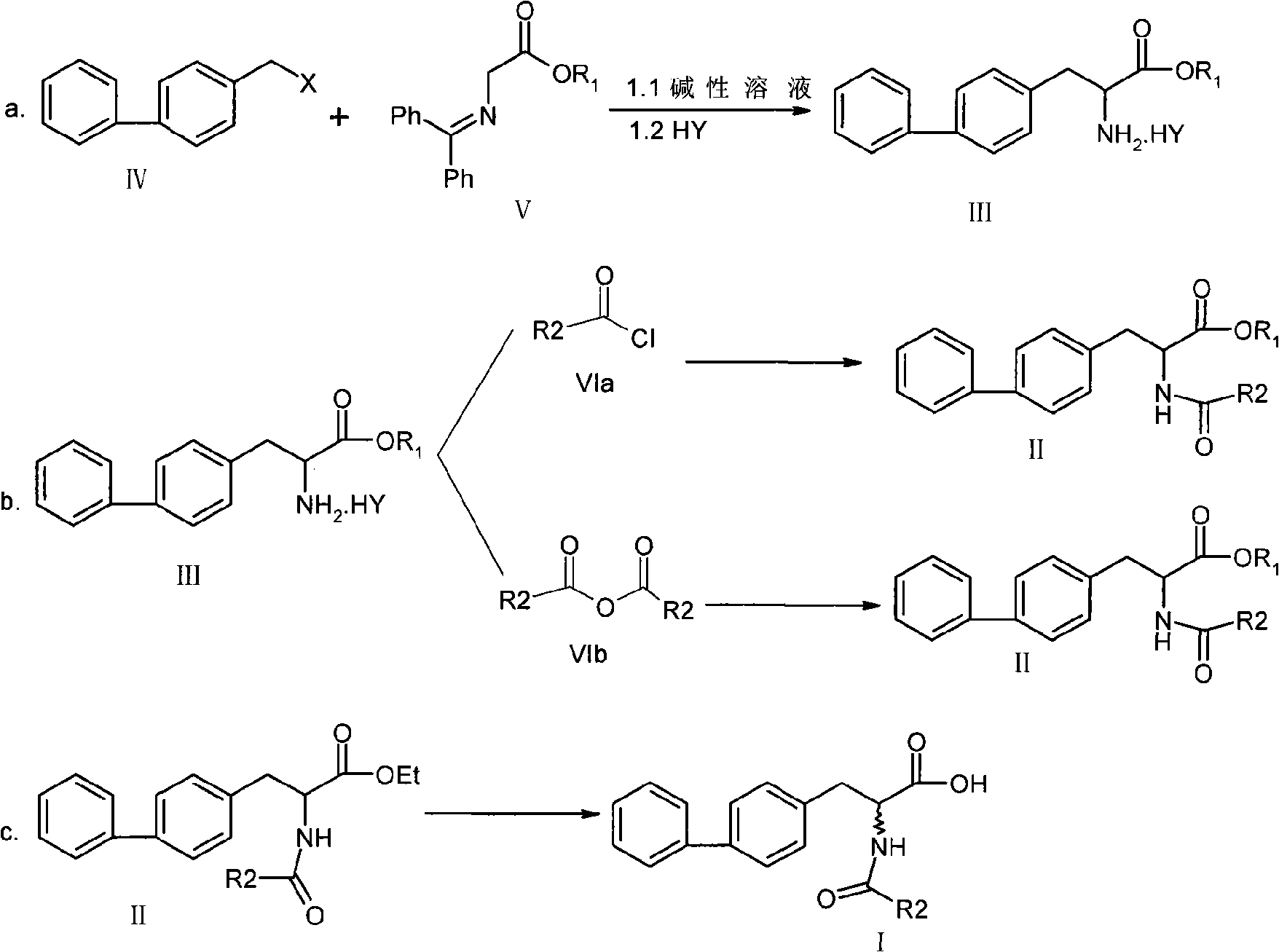
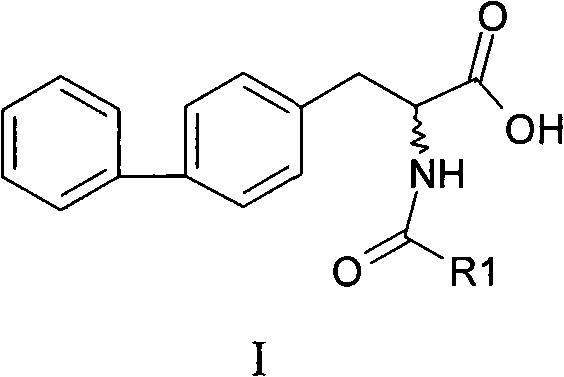
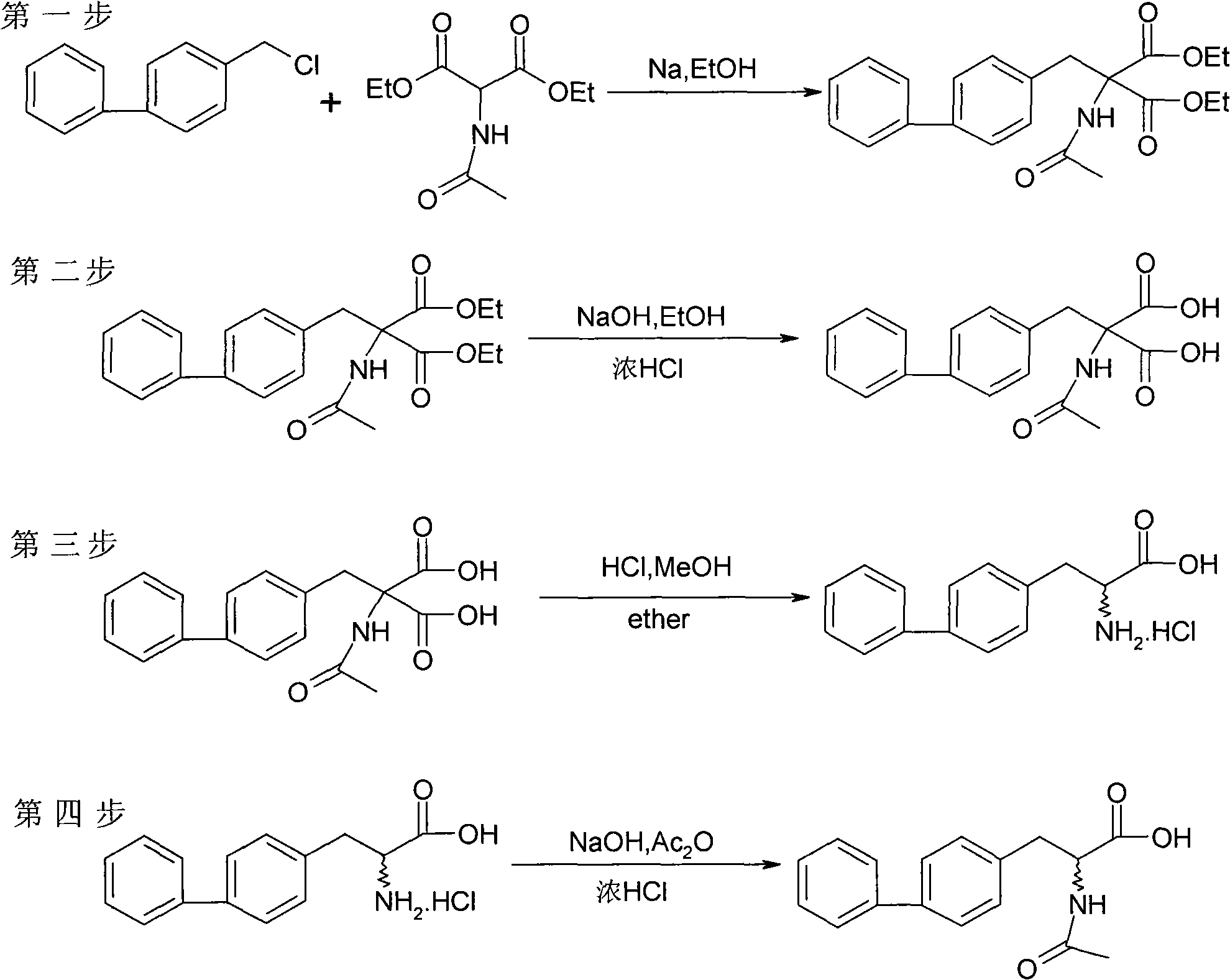
Chemical synthesis method of 2-acylamino-3-biphenyl propionic acid
A technology of biphenylpropionic acid and acylamino, which is applied in the field of chemical synthesis of 2-acylamino-3-biphenylpropionic acid, can solve the problem of low economy and practicability, low reagent selectivity, and long reaction route problems such as high reagent selectivity, short synthetic route and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 (2-amino-3-(biphenyl-4-yl) synthesis of methyl propionate)
[0044] In a clean flask, add 20.27g (100mmol) of 4-chloromethylbiphenyl, 25.33g (100mmol) of methyl N-(diphenylmethylenyl)aminoacetate, and 115mL of toluene. After the addition is complete, adjust the internal temperature to 0°C. Add the prepared 20% NaOH solution (containing 4.0 g (100.0 mmol) of NaOH) dropwise, and keep warm for 3 hours after the drop is completed. After the heat preservation is completed, add 200 mL of water dropwise, and then heat for 1 hour. After the heat preservation, add 20% hydrochloric acid solution (containing 5.47 g (150.0 mmol) of HCl) dropwise, heat for 1 hour, let stand, separate the water layer to obtain the organic layer, and concentrate under reduced pressure. To dryness, 24.80 g of methyl 2-amino-3-(biphenyl-4-yl)propionate hydrochloride was obtained, and the product yield was 85%.
Embodiment 2
[0045] Example 2 (synthesis of 2-amino-3-(biphenyl-4-yl) propionic acid methyl ester salt)
[0046] In a clean flask, add 24.73g (100mmol) of 4-bromomethylbiphenyl, 25.33g (100mmol) of methyl N-(diphenylmethylenyl)aminoacetate, and 180mL of chlorobenzene. After adding, adjust the internal temperature to 0°C. Add the prepared 20% KOH solution (containing 5.61 g (100.0 mmol) of KOH) dropwise, and keep warm for 2 hours after the drop is completed. After the heat preservation is completed, add 200 mL of water dropwise, and then heat for 1 hour. After the heat preservation, add 20% formic acid solution (containing 9.21 g (200.0 mmol) of formic acid) dropwise, heat for 1 hour, let stand, separate the water layer to obtain the organic layer, and concentrate under reduced pressure To dryness, 26.52 g of methyl 2-amino-3-(biphenyl-4-yl)propionate formate was obtained, and the product yield was 88%.
Embodiment 3
[0047] Embodiment 3 (the synthesis of 2-amino-3-(biphenyl-4-yl) methyl propionate oxalate)
[0048] In a clean flask, add 29.41g (100mmol) of 4-iodomethylbiphenyl, 30.40g (120mmol) of methyl N-(diphenylmethenyl)aminoacetate, and 330mL of tetrahydrofuran. After the addition is complete, adjust the internal temperature to -5°C. Drop into the prepared 20% K 2 CO 3 solution (with K 2 CO 327.64g (200.0mmol)), after dropping, keep warm for 0.5h. After the heat preservation is completed, add 200 mL of water dropwise, and then heat for 1 hour. After the heat preservation, add 20% oxalic acid solution (containing 13.50 g (150.0 mmol) of oxalic acid) dropwise, heat for 1 hour, let stand, separate the water layer to obtain the organic layer, and concentrate under reduced pressure. To dryness, 28.53 g of methyl 2-amino-3-(biphenyl-4-yl)propionate oxalate was obtained, and the product yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com