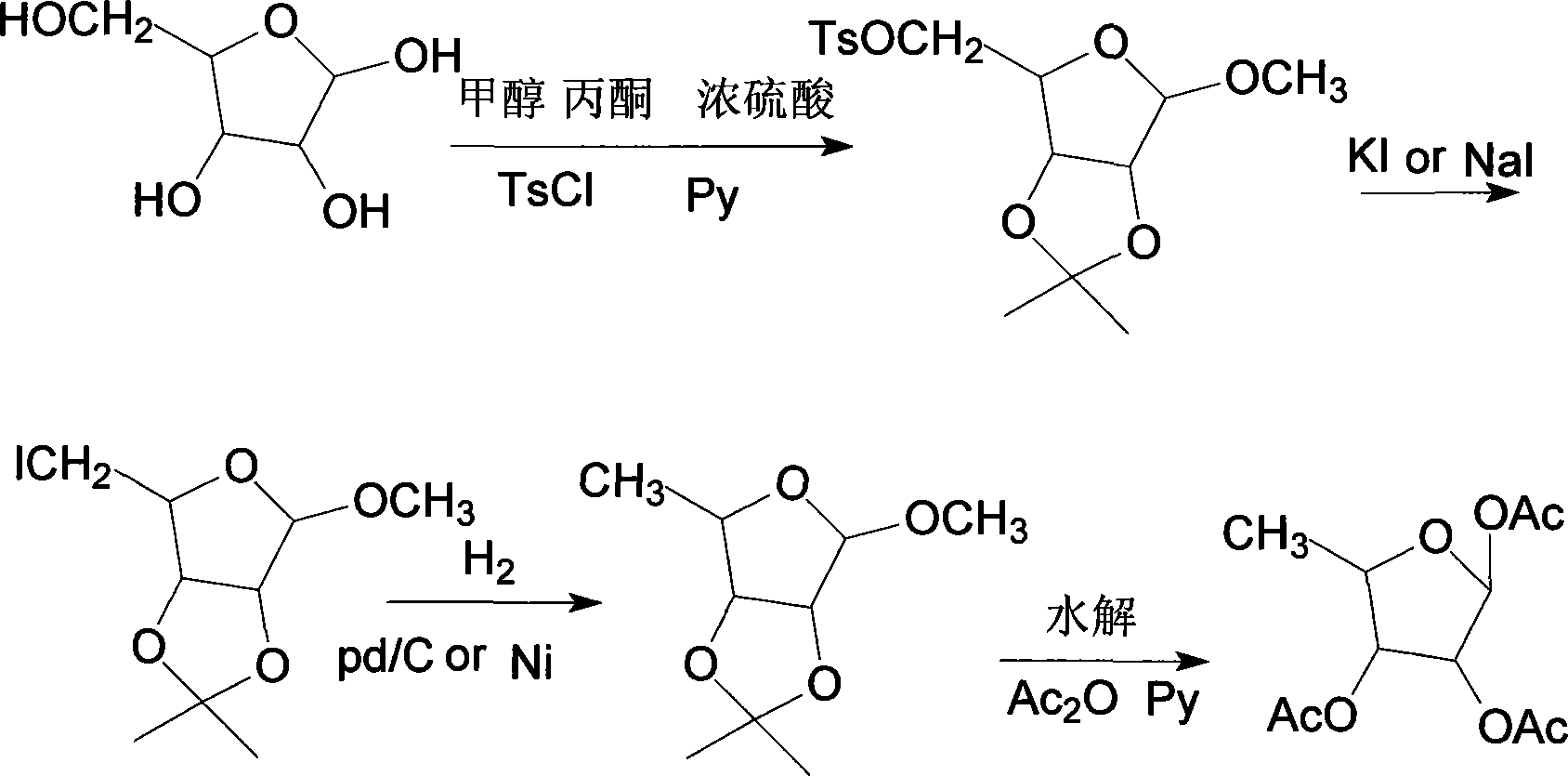
Method for synthesizing 1,2,3-O-tri-acetyl-5-deoxidation-D-ribose
A synthetic method and acetyl group technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve problems such as difficult crystallization, high cost, and high bond breaking temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] (1) Synthesis of 2,3-O-isopropylidene-5-O-tosyl-D-furanoside (I)
[0020] Add 150 g of ribose into a three-necked flask, add 5 times the amount of methanol and acetone respectively and stir at 40 ° C, adjust the pH to 3 to 4 with concentrated sulfuric acid, stir until clear, and detect the degree of reaction by TLC (ethyl acetate:petroleum ether 60~90℃ =9:1), after the reaction is over, add pyridine or triethylamine to adjust the pH to 6-7, stir for 20min, filter with suction, reclaim the solvent under reduced pressure to dryness, directly add 210g of toluenesulfonyl chloride for esterification in batches, and when the reaction ends, The reaction solution was poured into ice water to cool and crystallized to obtain 2,3-O-isopropylidene-5-O-p-toluenesulfonyl-D-furanoside, which was refined to obtain 289 g of white solid, mp 78-80°C, yield Rate 80%.
[0021] (2) Synthesis of 2,3-O-isopropylidene-5-iodo-5-deoxy-D-furanoside (II)
[0022] 289g of intermediate (I) was heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com