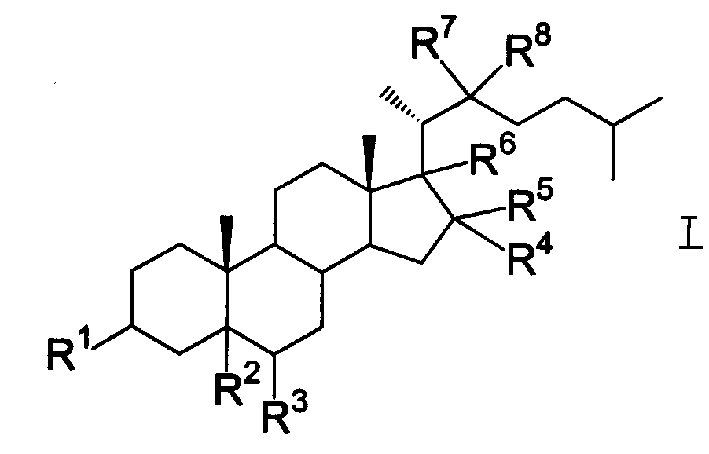
Steride, its systhesis method and use
A technology of steroidal compounds and synthetic methods, applied in the field of synthesizing natural product OSW-1 sapogenin, which can solve the problems of resource waste, serious environmental pollution, and obsolete degradation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 3β-hydroxyl-26,26-diphenylthiofurostane-5(6)-ene (14a)
[0043] Dissolve Diosgenin 10.000g (24.2mmol) in 100ml CH 2 Cl 2 In, add PhSH12.38ml (121.13mmol, 5.0eq), then slowly add BF 3 Et 2 O 6.11ml (48.6mmol, 2.0eq), stirred at room temperature until the raw material disappeared, added ethyl acetate to dilute, the organic phase was washed with 2N NaOH solution, and then washed with saturated NaCl solution until neutral, MgSO 4 Dry, filter, evaporate the solvent under reduced pressure, and separate by flash column chromatography to obtain 13.138 g (88.3%) of a white solid. C 39 h 52 o 2 S 2 , FW616; [α] D 17 =-45.1° (C=0.89, CHCl 3 ); mp 53-54°C; 1 H-NMR: 7.49-7.24 (10H, m, Ar), 5.37 (1H, d, J=4.2Hz, 6-H), 4.50 (1H, d, J=3.0Hz, 26-H), 4.27 (1H , m, 16-H), 3.52 (1H, m, 3-H), 3.24 (1H, m, 22-H), 1.17 (3H, d, J=6.6Hz, 21-Me), 1.05 (3H, s, 19-Me), 0.95 (3H, d, J=6.6Hz, 27-Me), 0.80 (3H, s, 18-Me); MS (EI): 507 (M + -109, 66.9%), 271 (1...
Embodiment 2
[0044] Example 2 Synthesis of 3β-hydroxyfurostane-5(6)-ene (15)
[0045] Dissolve 11.000g of 14a in 440ml of anhydrous EtOH, add about 30g of W-2 type Raney Ni, reflux until the raw material disappears, filter, the residue is fully washed with anhydrous EtOH, and the filtrate is spin-dried to obtain 6.653g (93.1%) of a white solid . C 27 h 44 o 2 , FW 400; [α] D 17 =-61.7° (C=1.01, CHCl 3 ); mp 69-70°C; 1 H-NMR: 5.34 (1H, d, J = 5.4Hz, 6-H), 4.30 (1H, m, 16-H), 3.52 (1H, m, 3-H), 3.31 (1H, m, 22- H), 1.02 (3H, s, 19-Me), 0.99 (3H, d, J = 7.2Hz, 21-Me), 0.89 (6H, d, J = 6.6Hz, 26, 27-Me), 0.81 ( 3H, s, 18-Me); MS (EI): 400 (M + ), 271 (100%); IR: 3381, 1052.
Embodiment 3
[0046] Example 3 Synthesis of 6β-acetoxy-3α, 5α-cyclofurostane (16)
[0047] Dissolve 1.111g of 15 in 5ml of dry pyridine, add 2.448g of TsCl under ice-cooling, react at room temperature until the raw material disappears, add ethyl acetate to dilute, wash the organic phase four times with saturated NaCl solution, MgSO 4 Dry, filter, and evaporate the solvent under reduced pressure. The obtained crude product was dissolved in 200ml of acetone, 3.5g of KOAc was added in 10ml of aqueous solution, refluxed until the raw material disappeared, the organic solvent was evaporated under reduced pressure, the organic matter was extracted with ethyl acetate, the organic phase was washed with saturated NaCl solution, MgSO 4 Dry, filter, and evaporate the solvent under reduced pressure. The obtained crude product was dissolved in 10ml Ac 2 O and 1ml of pyridine, add a small amount of DMAP, react at room temperature for 4h, and the reaction is complete. Add saturated NaHCO 3 solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com