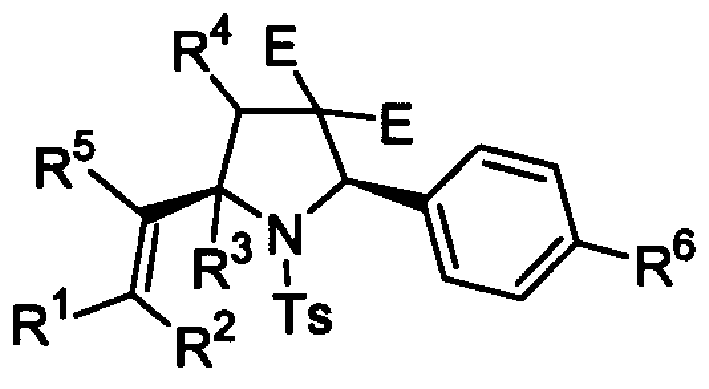
2,5-cis-bisubstituted pyrrolidine derivative and its preparing process and usage
A synthetic method, 5-cis-technology, applied in 2 fields, to achieve the effect of maintaining atom economy, easy availability of raw materials, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1c
[0013] Example 1 cis-1-p-toluenesulfonyl-2-phenyl-3,3-dicarboxymethyl-5-(1'-styryl)pyrrolidine: Take a reaction tube and add 138 mg of carbonic acid under nitrogen protection Potassium, 15mg Pd(PPh 3 ) 4 , 78mg imine C 6 h 5 =N-Ts, add 2 mL of tetrahydrofuran solvent, then add 46 mg of dimethyl 2-(2',3'-alkenyl)malonate, and 61 mg of iodobenzene. React at 85°C for 6 hours, filter, and purify by column chromatography (n-hexane: ether = 5: 1) to obtain 111 mg of 1-p-toluenesulfonyl-2-phenyl-3,3-dicarboxylate-5-( 1'-Styryl)pyrrolidine (cis / trans>99:1), yield 86%. 1 H NMR (CDCl 3 , 300MHz): δ=7.53(d, J=8.29Hz, 2H), 7.30-7.41(m, 5H), 7.11-7.28(m, 7H), 5.86(s, 1H), 5.36(s, 1H), 5.25(s, 1H), 4.50(dd, J=11.68, 5.98Hz, 1H), 3.65(s, 3H), 3.15(s, 3H), 2.84(dd, J=13.70, 11.68Hz, 1H), 2.60 (dd, J=13.70, 5.98Hz, 1H), 2.39(s, 3H); MS (70eV): m / z (%): 520 (14.32) [M + +1], 364(100); IR(KBr): ν=1749, 1729, 1635, 1597, 1493, 1350, 1165cm -1 ;Elemental analysis: ...
Embodiment 2c
[0014] Example 2cis-1-p-toluenesulfonyl-2-phenyl-3-cyano-3-methylcarboxylate-5(1'-(4'-propylphenyl)-2'-propylpentene base) pyrrolidine: take a reaction tube, add 159mg sodium carbonate, 25mg Pd(PPh 3 ) 4 , 78mg imine C 6 h 5 =N-Ts, add 1,4-dioxane solvent 2mL, then add 59mg 2-(4'-propyl-2', 3'-pentadienyl) ethyl cyanoacetate, and 92mg p-propyl iodobenzene. Reacted at 85°C for 15 hours, filtered, and purified by column chromatography (n-hexane: ether = 5:1) to obtain 124 mg of 1-p-toluenesulfonyl-2-phenyl-3-cyano-3-methylcarboxylate-5 -(1'-(4'-propylphenyl)-2'-propylpentenyl)pyrrolidine (cis / trans>95:5), yield 81%. 1 HNMR (CDCl 3 , 300MHz): δ=7.23-7.75(m, 13H), 5.40(s, 1H), 4.39(dd, J=11.83, 5.70Hz, 1H), 3.67(s, 3H), 2.48-2.76(m, 4H ), 2.36(s, 3H), 2.26(t, J=6.97Hz, 4H), 1.56-1.70(m, 2H), 1.28-1.51(m, 4H), 0.86-0.99(m, 9H); MS( 70 eV): m / z (%): 612 (6.54) [M + ], 91(100); IR(KBr): ν=2262, 1773, 1742, 1640, 1355, 1168 cm -1 ;Elemental analysis: ...
Embodiment 3c
[0015] Example 3 cis-1-p-toluenesulfonyl-2-phenyl-3,3-dicarboxymethyl-5-(1'-(4"-methylstyryl)pyrrolidine: take a reaction tube, Add 138mg potassium carbonate, 15mg Pd(PPh 3 ) 4 , 194mg imine C 6 h 5 =N-Ts, add 2 mL of tetrahydrofuran solvent, then add 46 mg of dimethyl 2-(2',3'-alkenyl)malonate, and 66 mg of p-methyliodobenzene. React at 85°C for 12 hours, filter, and purify by column chromatography (n-hexane: ether = 5: 1) to obtain 122 mg of 1-p-toluenesulfonyl-2-phenyl-3,3-dicarboxylate-5-( 1'-(4'-methylstyryl)pyrrolidine (cis / trans>99:1), yield 92%. 1 H NMR (CDCl 3 , 300MHz): δ=7.46(d, J=8.40Hz, 2H), 7.25(d, J=8.40Hz, 2H), 7.04-7.22(m, 9H), 5.79(s, 1H), 5.24(s, 1H), 5.14(s, 1H), 4.41(dd, J=11.53, 5.86Hz, 1H), 3.57(s, 3H), 3.07(s, 3H), 2.76(dd, J=13.67, 11.53Hz, 1H ), 2.52 (dd, J=13.67, 5.86Hz, 1H), 2.32 (s, 3H), 2.31 (s, 3H); MS (70 eV): m / z (%): 533 (7.55) [M + ], 416(100); IR(KBr): ν=1771, 1746, 1646, 1597, 1511, 1350, 1162 cm -1 ;E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com