Patents
Literature
33 results about "Benzopyrrole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
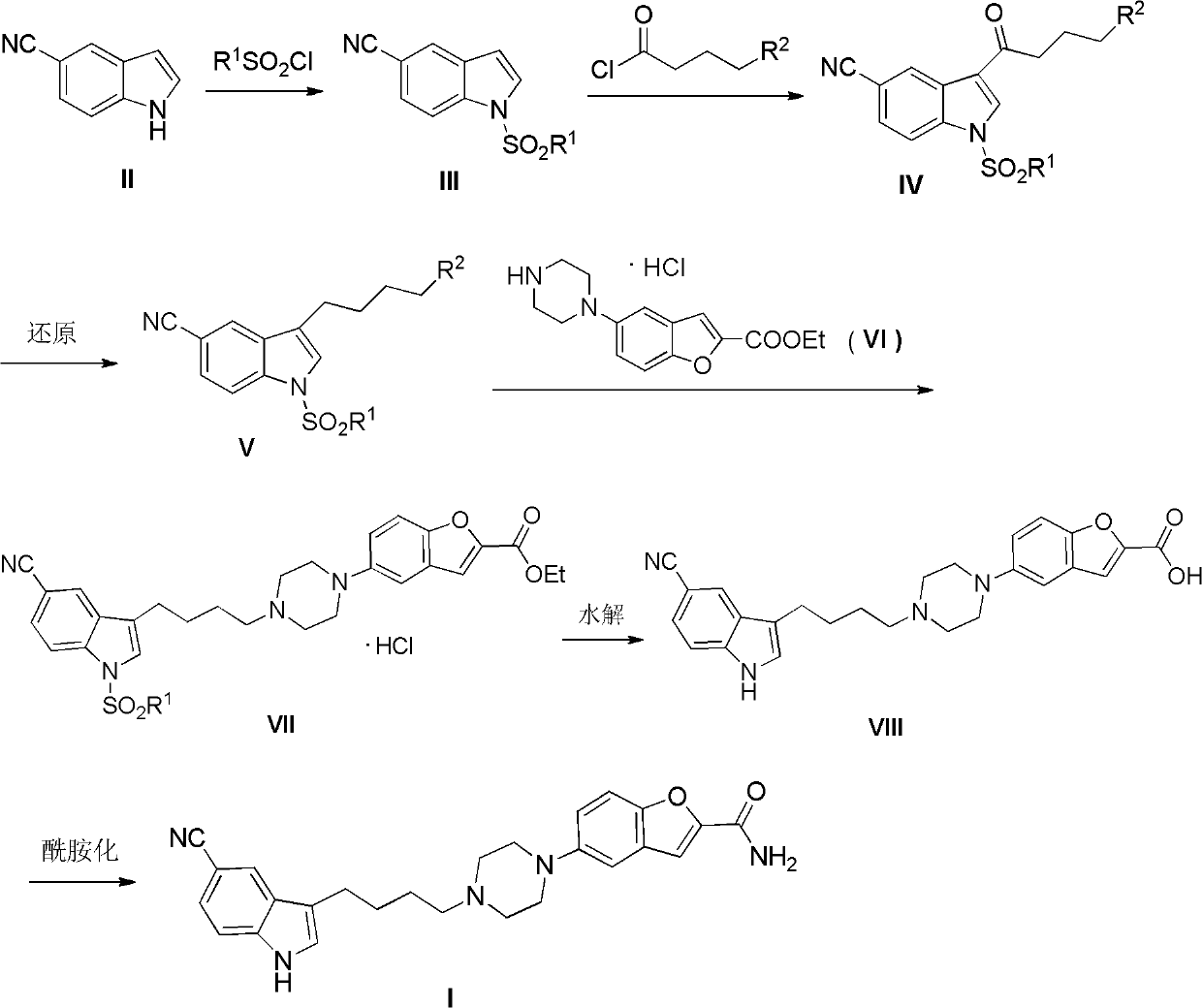
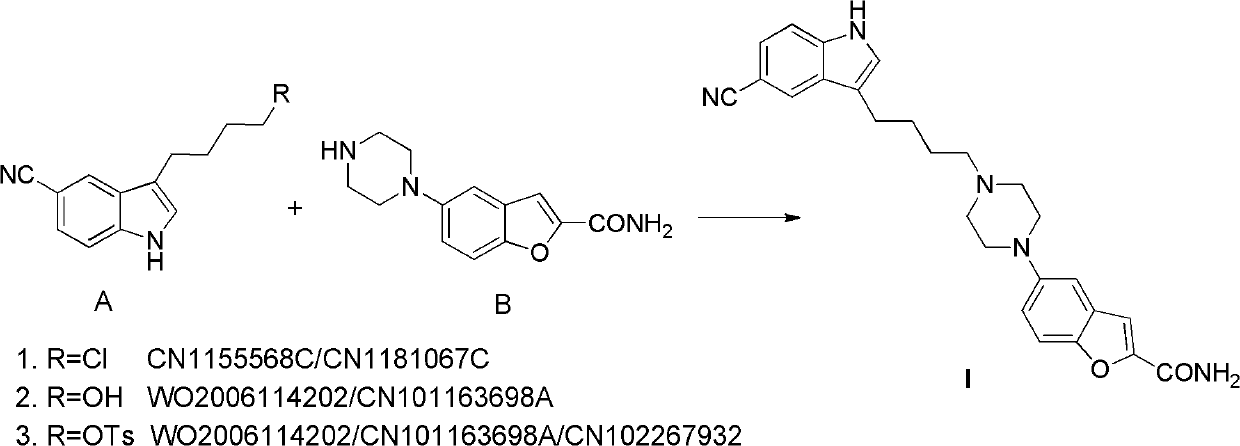
Preparation method of antidepressant drug-vilazodone
InactiveCN103304547AReduce usageReaction raw materials are readily availableOrganic chemistryCarboxylic acidMethyl group
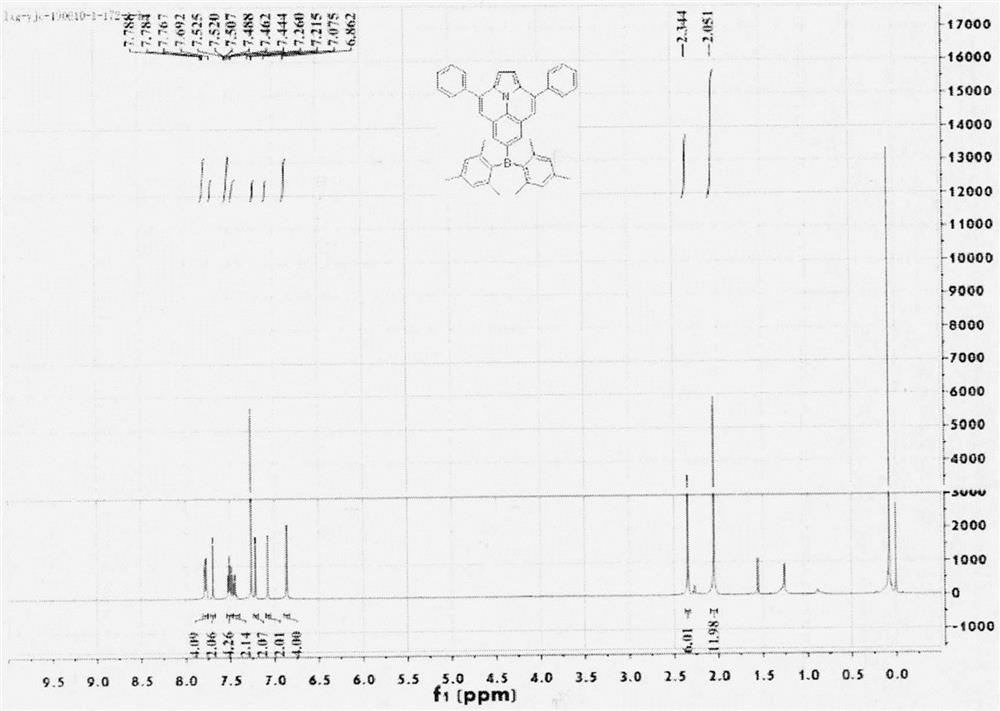
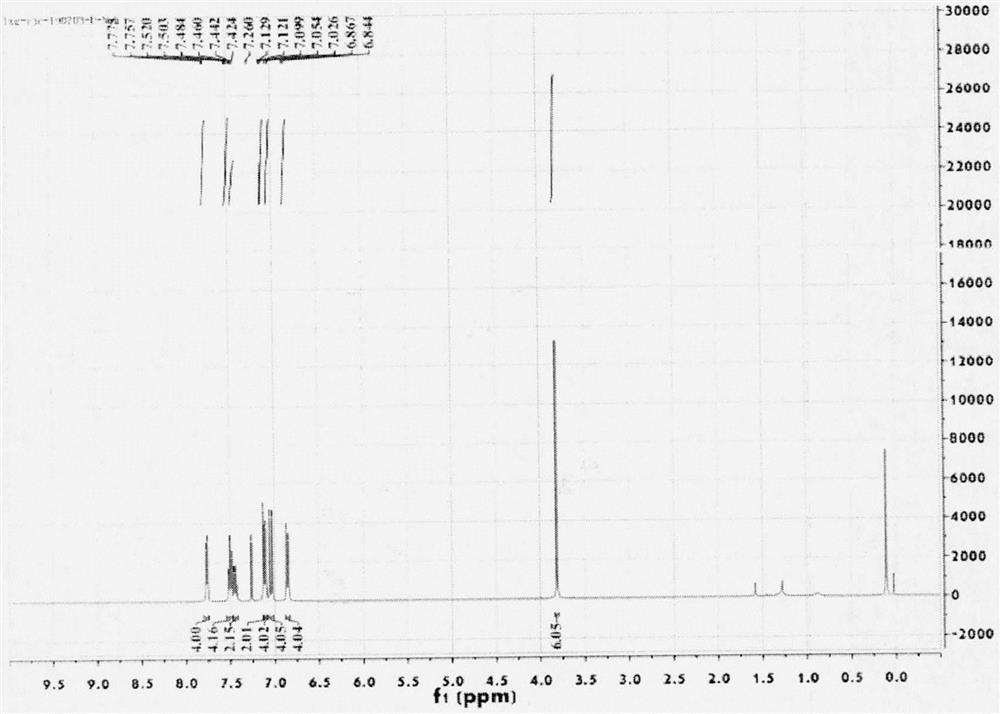
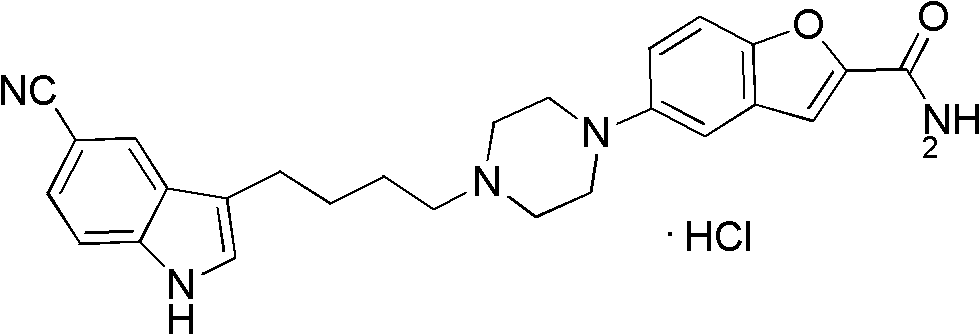
The invention relates to the field of drug synthesis, and in particular relates to a preparation method of an antidepressant drug-vilazodone. The preparation method is characterized by comprising the following steps of: protecting a 1-location nitrogen atom of 5-cyano benzopyrrole used as an initial raw material through utilizing toluenesulfonyl, carrying out 3-location Friedel-Carfts acylation, and selectively reducing carbonyl into methylidyne so as to obtain 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole; reacting the 1-tosyl-3-(4-chlorobutyl)-5-cyano benzopyrrole with 5-(piperazine-1-radical) benzofuran-2-nonanoic acid-ethyl ester hydrochloride, hydrolyzing through one step, and removing tosyl and ethyl at the same time so as to obtain 5-(4-(4-(5-cyan-1H-benzopyrrole-3-radical) butyl) piperazine-1-radical) benzofuran-2-carboxylic acid; finally, amidating so as to obtain vilazodone, and salifying vilazodone with hydrogen chloride so as to obtain hydrochloric acid vilazodone. The method can be used for overcoming a plurality of defects of a conventional synthetic method, and has the advantages of easiness in raw material acquisition, high yield, good selectivity, convenience in operation, suitability for industrial production and high application value.
Owner:CHINA PHARM UNIV
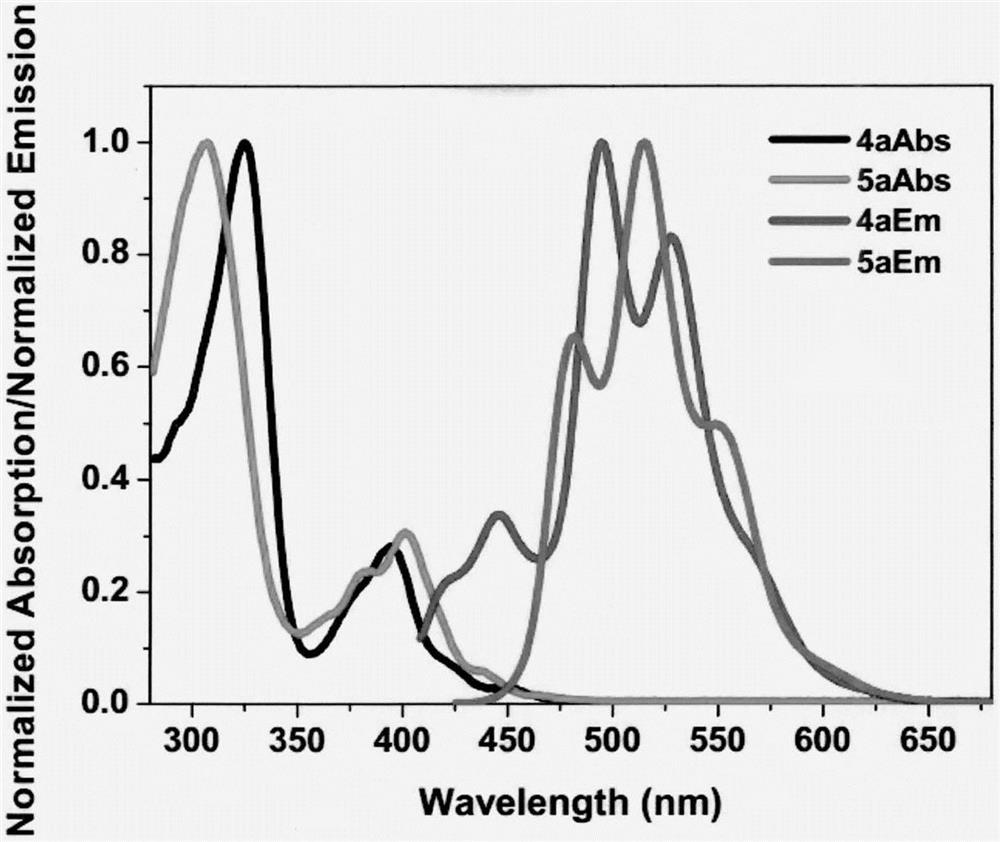
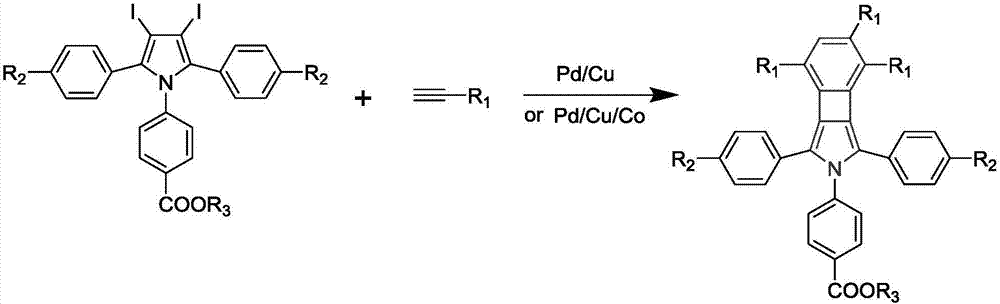
Cyclobutadiene benzopyrrole derivative with AEE effect and preparation thereof
InactiveCN107011244AHigh emission wavelengthEasy to operateOrganic chemistryLuminescent compositionsBenzeneOrganic synthesis
The invention relates to a cyclobutadiene benzopyrrole derivative with an AEE effect and preparation thereof and belongs to the technical field of organic synthesis. The cyclobutadiene benzopyrrole derivative is a benzo condensed ring derivative with pyrrole-cyclobutadiene-benzene ring combined in a large pi conjugated structure, and has an AEE property; the light-emitting wavelengths are higher than 550nm, the light is located in an orange light region or a red light region, and the derivative can be used for synthesizing an infrared or far infrared organic fluorescent dye. The method of preparing the cyclobutadiene benzopyrrole derivative is a one-pot method which mainly comprises the steps of performing continuous Sonogashira reaction and cyclotrimerization reaction on a pyrrole derivative iodated in sites 3 and 4 to form an aromatic pi system with a five membered ring combined with a four membered ring and a six membered ring. The method is simple to operate and the used raw materials are cheap and easily available.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Compound, organic light-emitting device and display device
InactiveCN108218867AImprove luminous efficiencyReduce the driving voltageOrganic chemistrySolid-state devicesOrganic light emitting deviceDisplay device
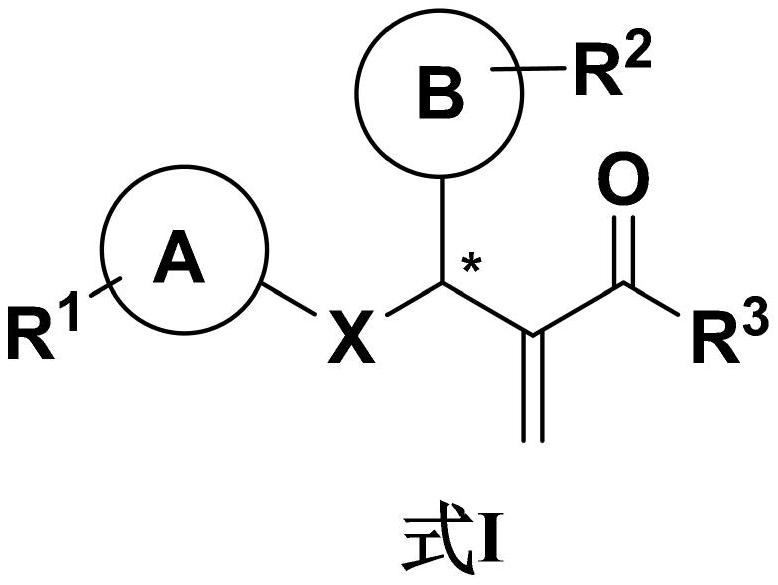
The invention relates to the field of display and especially relates to compound, an organic light-emitting device and a display device. The compound is shown as a formula I in the following image description, wherein X group is chosen from carbazolyl, dihydroacridine, dihydrophenazine, phenoxazine, phenothiazine, benzopyrrolocarbazolyl, benzofurocarbazolyl, benzothienocarbazolyl, indenocarbazolylor triphenylamine; the X perssadgroup is substituted or unsubstituted; A group is chosen from acryl with a carbon atom number as 6 to 30, alkyl with a carbon atom number as 1 to 20 or cycloalkyl witha carbon atom number as 3 to 20; the A group is substituted or unsubstituted.
Owner:FUYANG XINYIHUA MATERIAL TECH
Hydroxyl radical ratio type fluorescent probe as well as preparation method and application thereof
ActiveCN111072699AHigh selectivityHigh sensitivityGroup 3/13 element organic compoundsColor/spectral properties measurementsFluoProbesArtemisinins
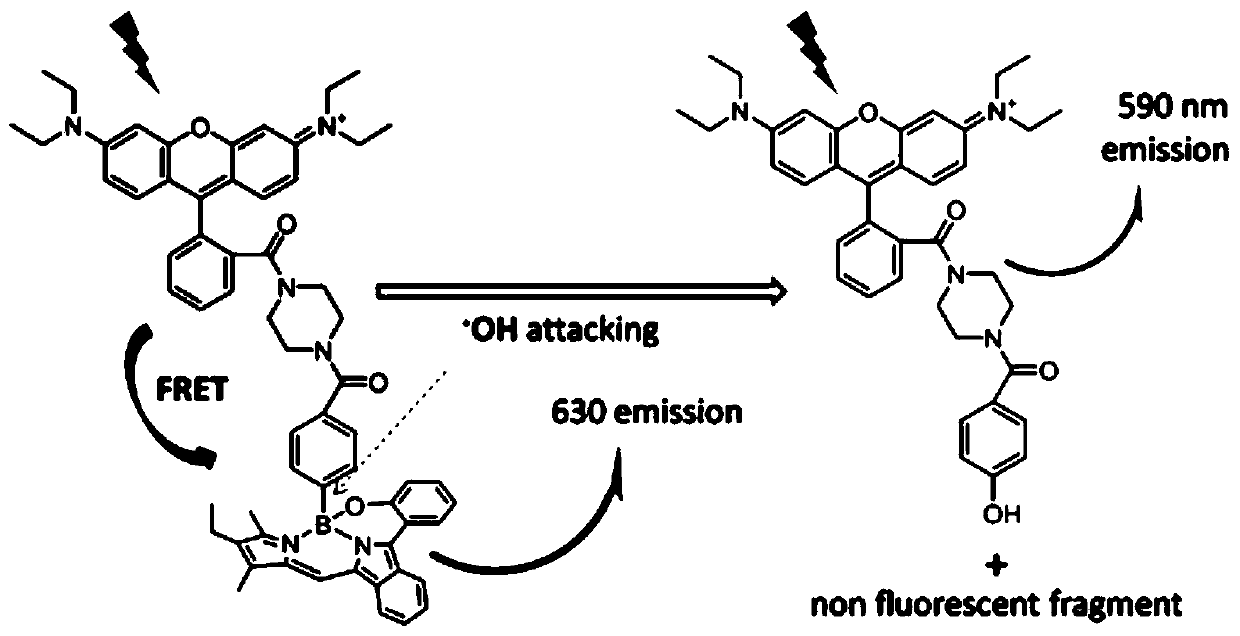
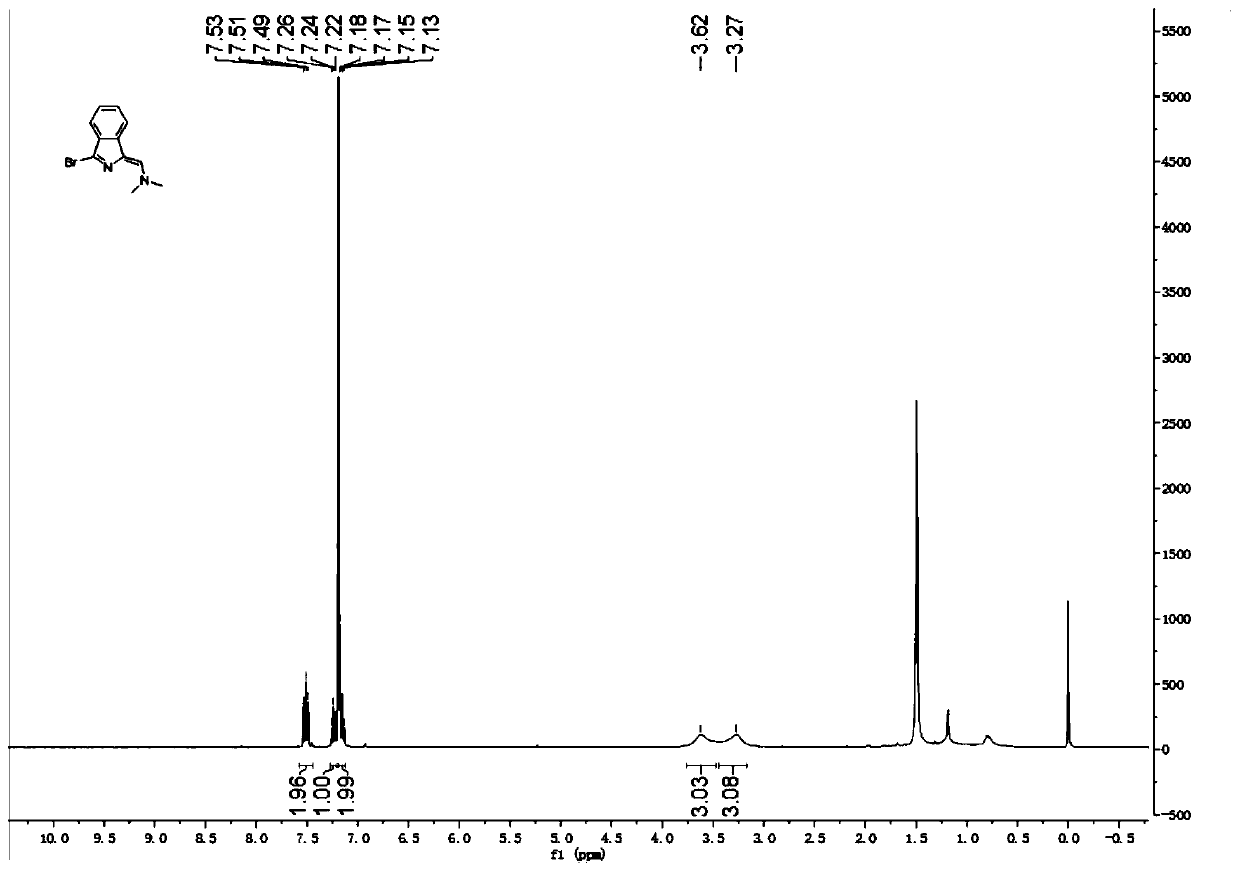
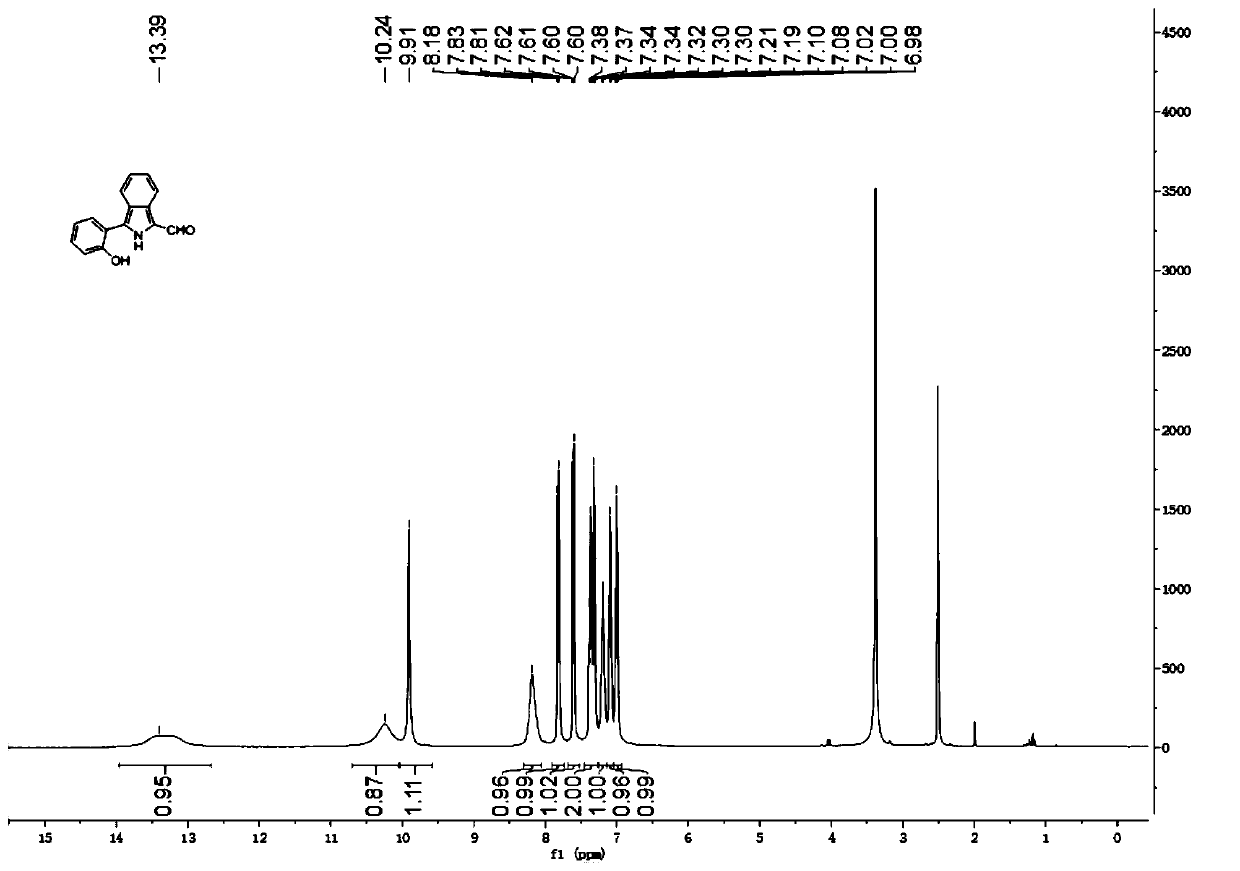
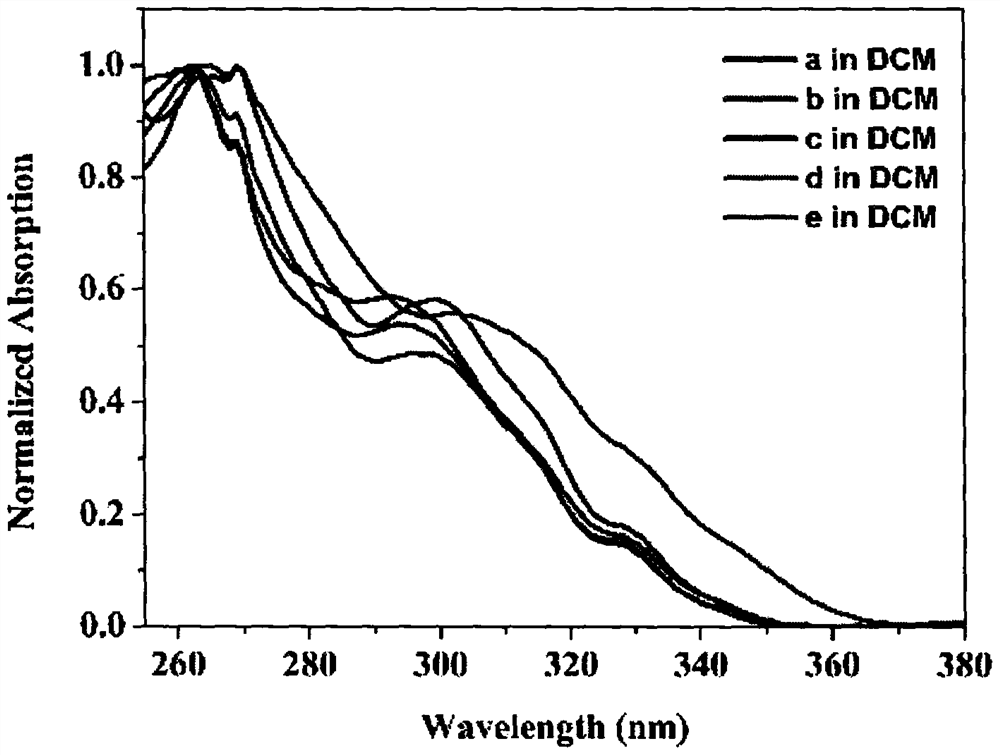
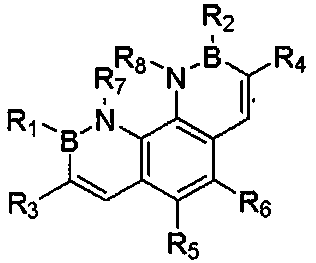
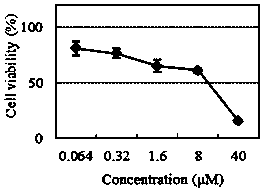
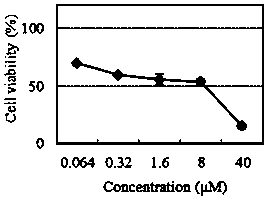
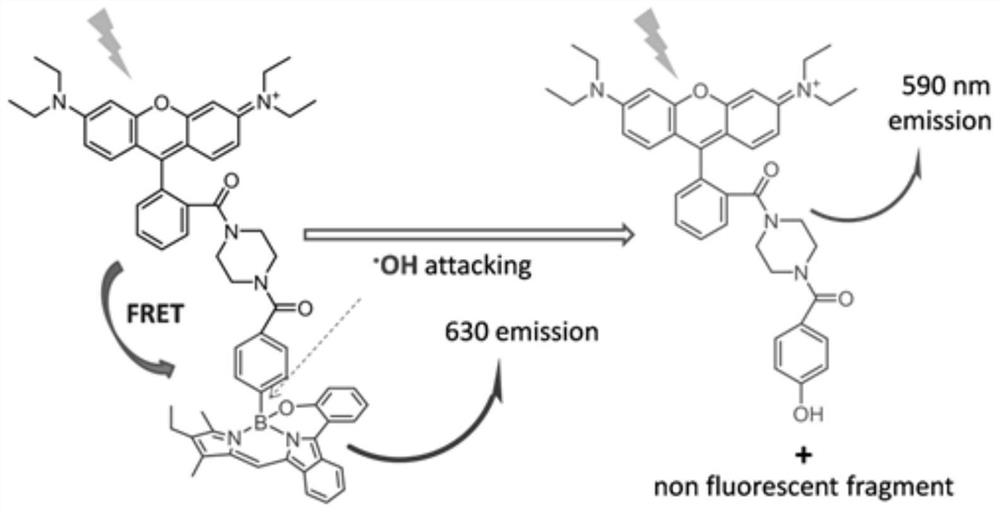
The invention relates to a novel fluorescence resonance energy transfer (FRET) probe Rho-Bob, which is constructed by connecting rhodamine B (Rho) with an N2O type benzopyrrole boron complex (Bobpy),and is used for OH ratio type fluorescence detection and imaging. Rho-Bob shows the characteristic of being sensitive to the hydrophilicity / hydrophobicity of the environment, and has excellent mitochondrial localization capability. Rho-Bob is successfully applied to intracellular .OH ratio type fluorescence imaging. The .OH can be generated by a Fenton reaction, and can also be generated by activation of intracellular drugs. The Rho-Bob probe has high selectivity and sensitivity to hydroxyl radicals, and the detection limit is as low as a nanomole level (680nM). According to the invention, .OHgenerated by artemisinin molecules in cell mitochondria is observed for the first time by using Rho-Bob, and endogenous hydroxyl radicals are found to exist in zebra fish gastrointestinal tracts (GI)under normal culture conditions for the first time. The invention not only provides the practical probe for .OH detection and imaging, but also provides an important thought for constructing novel ratiometric probes of other ROS.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Furan fused boron aza dihydropyrene and synthesis method thereof
The invention aims to provide a design and synthesis method of a furan fused boron aza dihydropyrene polycyclic aromatic hydrocarbon molecule, and provides more schemes for obtaining more efficient organic photoelectric materials. In order to achieve the purpose, the technical scheme adopted by the invention is as follows: 3, 6-bis (furan-3-yl) benzene-1, 2-diamine is taken as a raw material, the furan fused boron aza dihydropyrene large conjugated polycyclic aromatic hydrocarbon and the derivative thereof at a specific position are generated through nitrogen guidance, and the structural formula of the compound is shown in the specification,wherein the R is independent,can be alkane and can also be a substituted or unsubstituted aryl group or heteroaryl group; R1 and R2 are independent, can be substituted or non-substituted aryl, heteroaryl, alkane, cycloalkane, olefin and alkyne, and can also be hydrogen or single substituted halogen atoms X: F, Cl, Br and I. and Ar aryl is a benzene ring, a thiophene ring, a furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzopyridine, a naphthalene ring, an anthracene ring, phenene, tetracene, pyrene, linear or angular pentacene, hexacene, indene or fluorene respectively.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
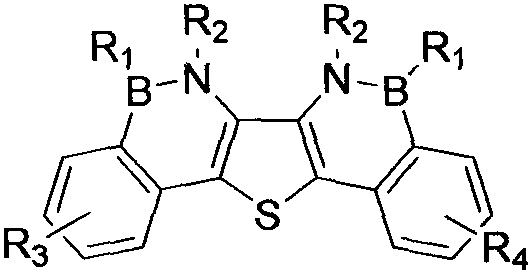
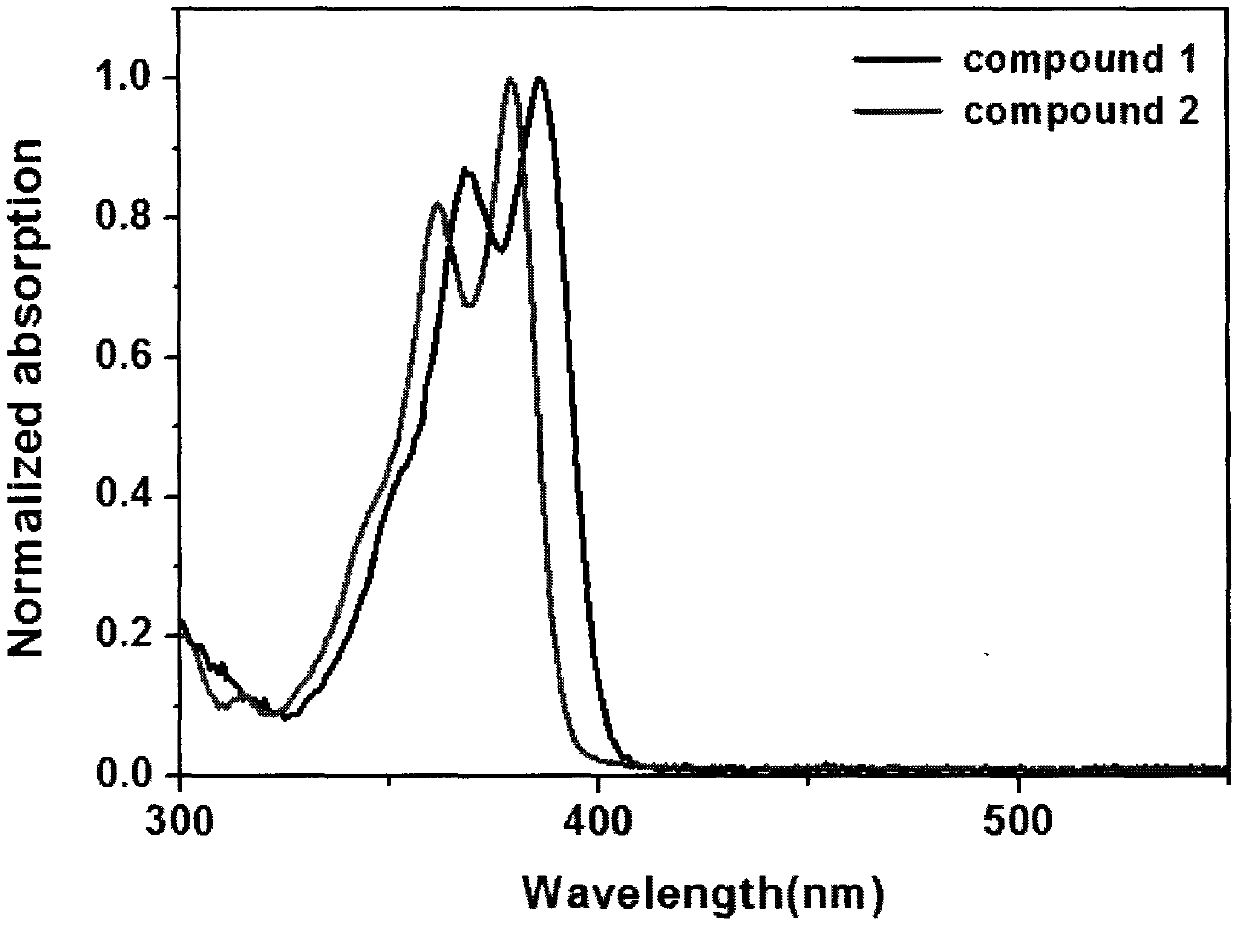
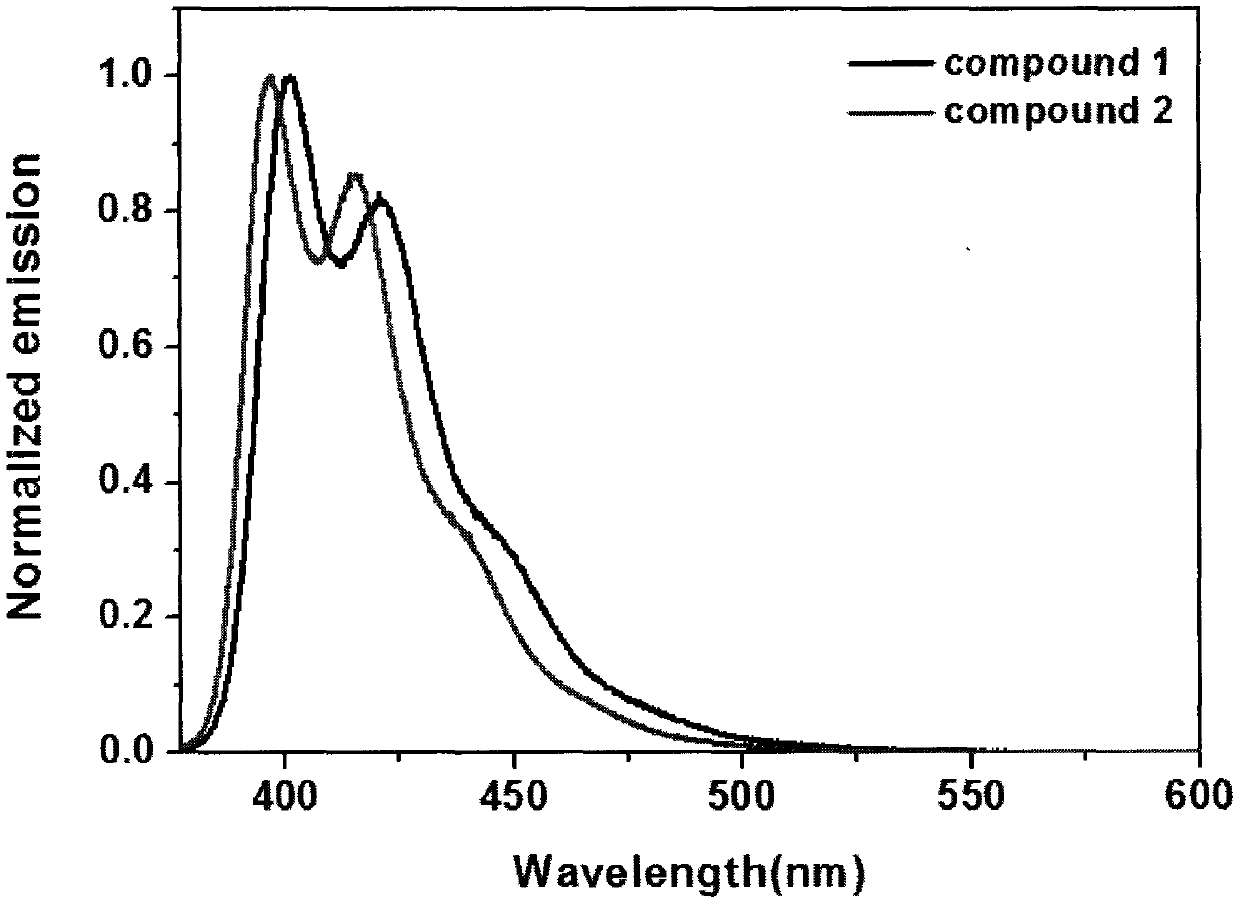
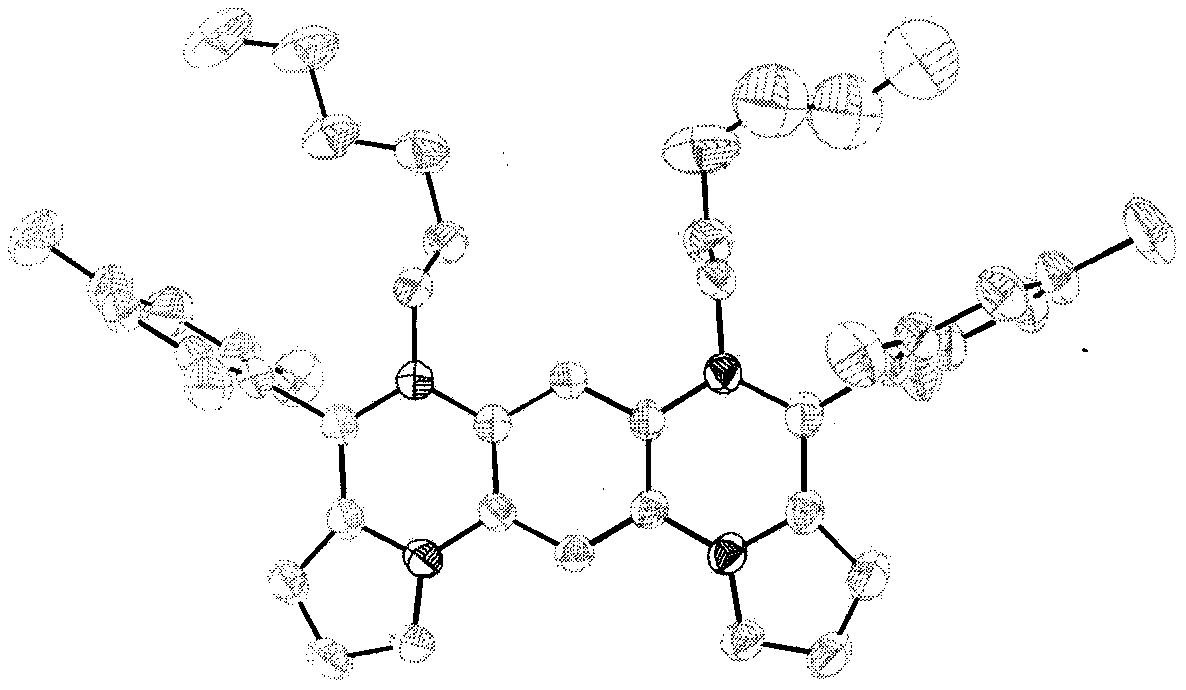
Synthesis method of boron aza-naphthalene thiophthene hetero arene and derivative thereof
InactiveCN109796480ASolid-state devicesSemiconductor/solid-state device manufacturingFuranSynthesis methods
The invention relates to a synthesis method of boron aza-naphthalene thiophthene hetero arene and a derivative thereof. The photophysical properties and single crystal diffraction structures of the compounds are tested, and the potential application value of the organic material in organic electrochemistry is further researched. According to the structural formula of the compound, R1, R2, R3 and R4 are independent substituted or non-substituted groups which comprise alkyl and aryl (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, quinoline,naphthalene ring, anthracene ring, phenalene, naphthacene, pyrene, a compound shown in the description, linear or angled pentacene, hexacene, indene, fluorene and the like). R3 and R4 can also be used as a single substituted halogen atom X : F, Cl, Br and I. The formula is shown in the description.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Synthetic method of bis-pyrrole fused boron naphthazine and derivatives of bis-pyrrole fused boron naphthazine
The invention relates to a synthetic method of bis-pyrrole fused boron naphthazine and derivatives of bis-pyrrole fused boron naphthazine. The photoelectric physical properties of the compounds are tested and studied, and study on application value of the organic materials in preparation of OLED devices is carried out. The structure formula of the compounds is disclosed in the invention, wherein R1, R2, R4, and R5 are respectively used for representing independent substituent groups or non-substituent groups including alkyl, aryl (phenyl ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, quinoline, naphthalene ring, anthracene nucleus, phenalene, tetracene, pyrene, linear or angled pentacene, hexacene, indene, fluorine, and the like), and R3 is used for representing alkyl, acyl, nitro, sulfonic group, aldehyde group, diazo group, or single substitution halogen atom X, F, Cl, Br, and I.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Synthetic method of boroazaphenanthrene and derivatives thereof
The invention relates to a synthetic method of boroazaphenanthrene and derivatives thereof, tests the photoelectric physical property of the compounds and further researches potential application value of the organic material in the aspect of organic electrochemistry. The structural formula of the compound is described in the specification, wherein R1, R2, R3 and R4 are respectively independent substituted or non-substituted groups, including alkyl groups and aryl groups (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzoylpyridine, naphthalene ring, anthracene ring, phenalene, naphthacene, pyrene, chrysene, linear or angular pentacene, hexacene, indene and fluorene and the like), wherein R1 and R2 also can be single substituted halogen atom X: F, Cl, Br and I.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
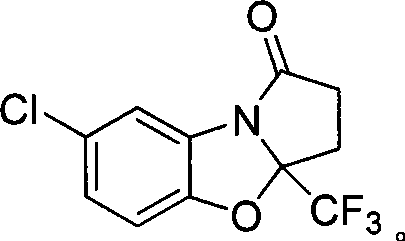
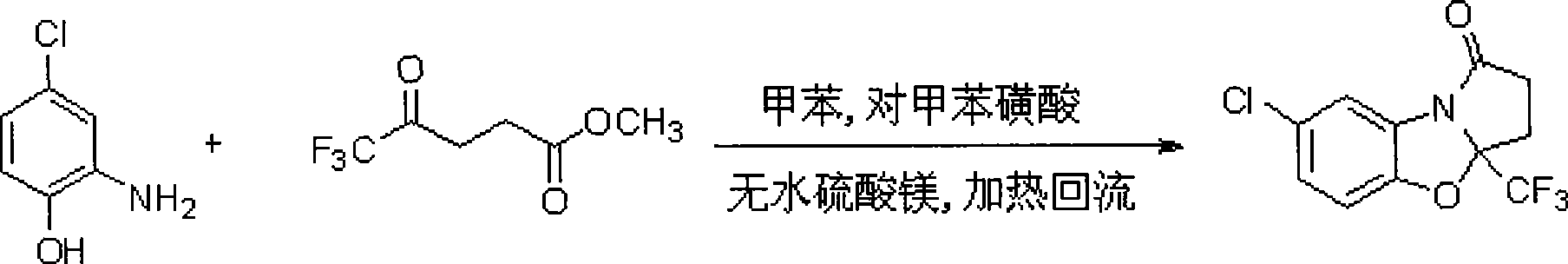
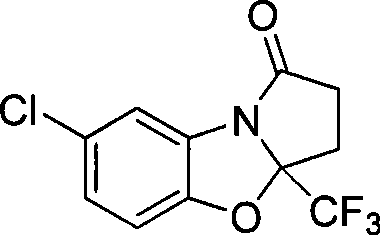
7-chlorine-3a-(trifluoromethyl)-3, 3a-dihydrobenzo (d) pyrrole (2, 1-b)-oxazole-1(2H)-ketone and synthetic method thereof
InactiveCN101531669AImprove stabilityImprove physiological activityOrganic chemistryKetonic acidsKetone
The invention relates to a 7-chlorine-3a-(trifluoromethyl)-3, 3a-dihydrobenzo (d) pyrrole (2, 1-b)-oxazole-1(2H)-ketone and a synthetic method thereof. The structural formula of the compound is shown as the right formula; the method comprises the following steps: trifluoro-gama-methyl ketonic acid and p-toluene sulfonic acid with the amount of a catalyst are dissolved in methylbenzene, anhydrous magnesium sulfate with the amount of the catalyst is added, reflux is carried out for 20 to 60 minutes under stirring and then 2-amino-4-chlorophenol is added; the obtained mixture continuously reacts for 10 to 15 hours, and then p-toluene sulfonic acid with the amount of the catalyst is added and reflux reaction is carried out for 10 to 15 hours; the molar ratio of trifluoro-gama-methyl ketonic acid to 2-amino-4-chlorophenol is (1-1.2):1, and then reaction is ended; and off-white solid is obtained by separation and purification, namely 7-chlorine-3a-(trifluoromethyl)-3, 3a-dihydrobenzo (d) pyrrole (2, 1-b)-oxazole-1(2H)-ketone. The 7-chlorine-3a-(trifluoromethyl)-3, 3a-dihydrobenzo (d) pyrrole (2, 1-b)-oxazole-1(2H)-ketone has stronger activity and is more beneficial to being absorbed. The invention has easily obtained raw materials and very simple operation, adopts one-pot synthesis, has productivity up to 68 percent and is applicable to production on large scale.
Owner:SHANGHAI UNIV
Synthesis method of diboron azatriphenylene and derivative thereof
InactiveCN110526935AOrganic chemistry methodsGroup 3/13 element organic compoundsSynthesis methodsStructural formula
The invention relates to a synthesis method of diboron azatriphenylene and a derivative thereof, tests the photoelectric physical properties of the compounds and further researches potential application values of the organic materials on the aspect of organic electrochemistry. The compound has a structural formula as shown in the specification, wherein each of R1, R2, R3, R4, R5, R6, R7 and R8 isan independent substituted or non-substituted group and comprises an alkyl and an aryl (a benzene ring, a thiophene ring, a furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzopyridine, a naphthalene ring, an anthracene ring, phenalene, naphthacene, pyrene, jue as shown in the specification, linear or angular pentacene, hexacene, indene, fluorene and the like), wherein R1, R2, R3 and R4 may also be single substituted halogen atom X: F, Cl, Br and I.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Application of benzothiazoles and benzopyrroles in the preparation of antitumor drugs
ActiveCN108653282BOrganic active ingredientsAntineoplastic agentsBULK ACTIVE INGREDIENTProliferation activity
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI +1
A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound
Owner:DONGHUA UNIV
Instant beef steak as well as preparation method and application thereof
PendingCN114698788AGood elasticity and chewinessBright colorMeat/fish preservation by heatingFood processingBiotechnologyMicroorganism
The invention discloses a preparation method of ready-to-eat beef steak, which comprises the following steps: unfreezing pre-frozen originally cut beef steak, wiping redundant blood on the surface of the unfrozen beef steak by using absorbent paper, then placing the beef steak in a superheated steam tray, then carrying out superheated steam treatment on the beef steak, rapidly cooling, and then loading into a sterile bag, and performing water bath sterilization, and finally performing vacuum-pumping treatment to obtain the instant beef steak. No additive is added in the making process of the beef steak, the beef steak is sterilized while the fragrance of the beef steak is increased through heat treatment, the content of benzopyrene generated by the method is lower than that of benzopyrene generated by other methods, and harm of microorganisms is effectively reduced through secondary sterilization on the basis. According to the scheme, the produced benzopyrrole is effectively reduced and is far lower than the national standard 5.0 mu g / kg, and the steak is tough and tender in taste, good in aroma stability and comprehensive in nutrition.
Owner:FUJIAN AGRI & FORESTRY UNIV
Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same
Copolycarbonate optical articles include polycarbonate compositions comprising: a copolycarbonate having: 2 to 60 mole percent benzo[c]pyrrolidone carbonate units, 2 to 90 mole percent refractory carbonate units, and optionally 2 to 60 mol% of bisphenol A carbonate units. The copolycarbonate has less than 100 ppm of each of benzo[c]pyrrolidone, high-heat bisphenol, and bisphenol A monomer, and less than 5 ppm of each ion, and is composed of monomers each having a purity of at least 99.6% preparation. The polycarbonate composition has a glass transition temperature of 200° C. to 200° C. and a yellowness index of less than 30.
Owner:SHPP GLOBAL TECH BV
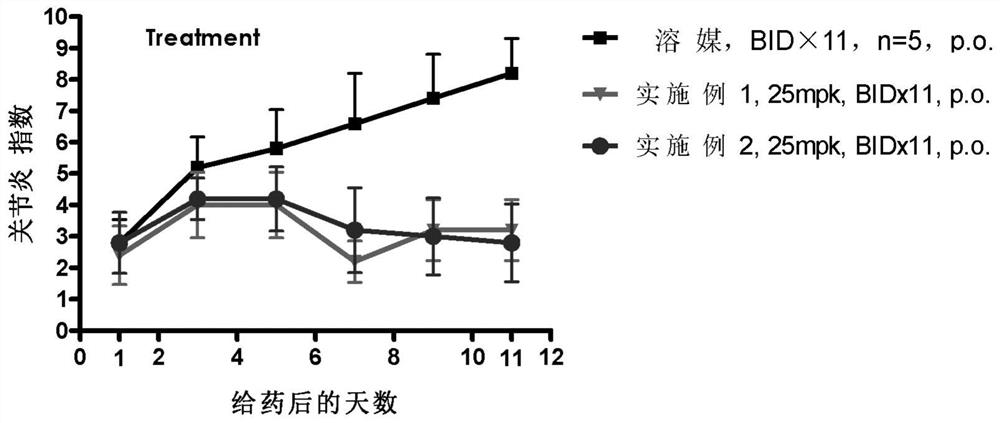
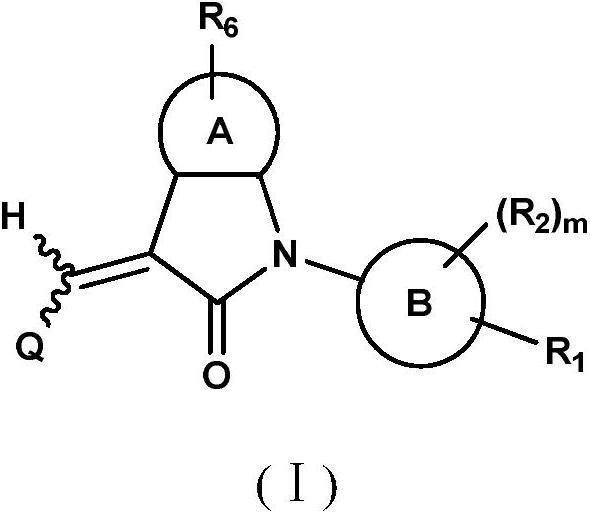
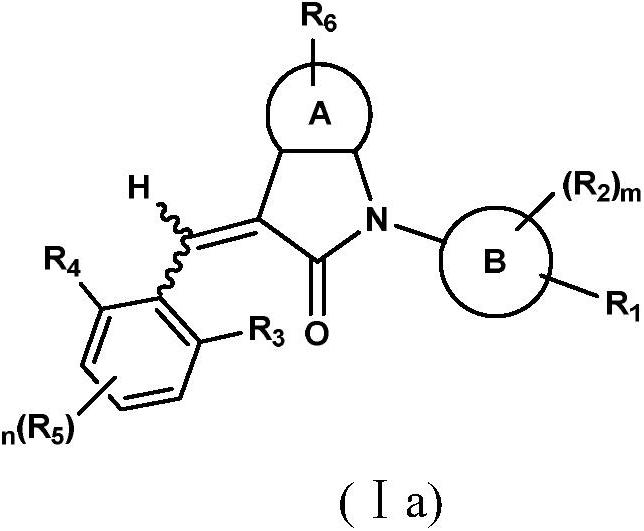
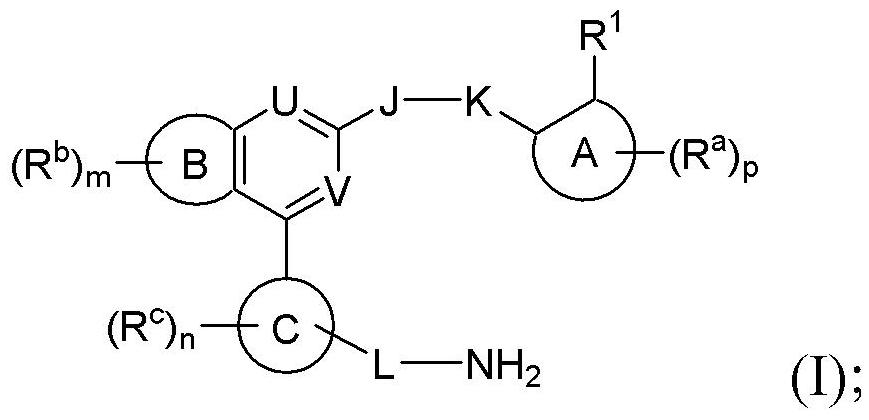
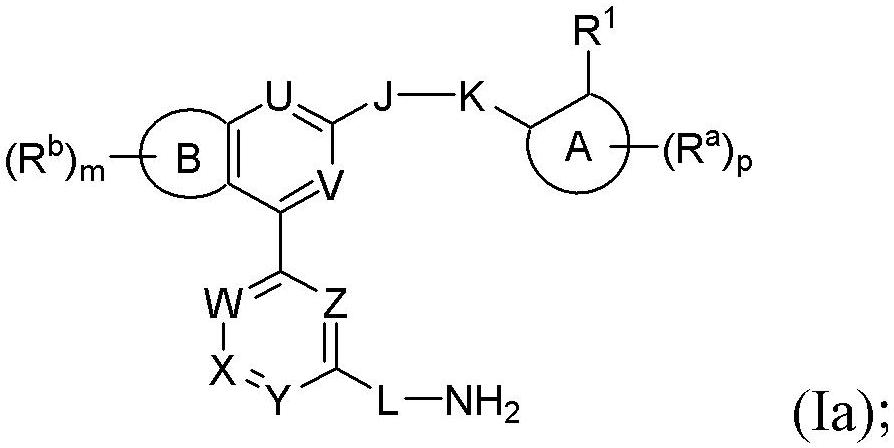
Benzopyrrolin-2-one derivatives substituted by n-benzoic acid group and use thereof
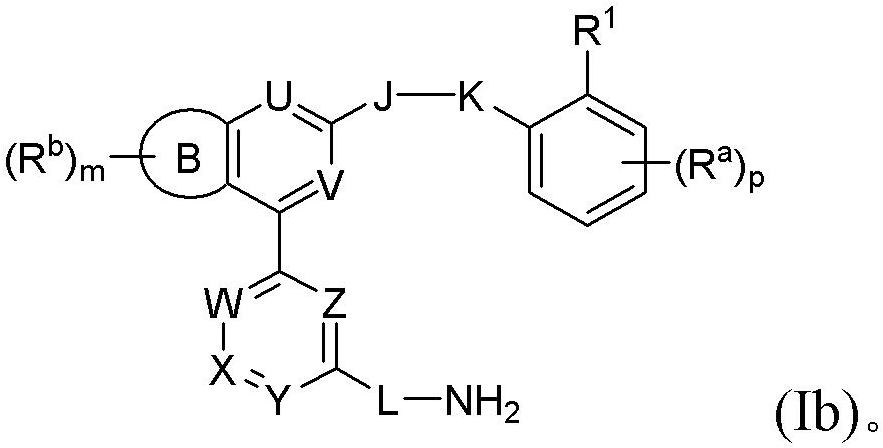
Disclosed are a compound, a stereisomer and a tautomer thereof, a pharmaceutically acceptable salt thereof, and a solvate or a prodrug thereof, which can be used for preventing or treating a ROR³ mediated disease. The compound has the structural formula (I).
Owner:BEIJING HANMI PHARMA CO LTD
6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same
InactiveCN101544645AImprove stabilityImprove physiological activityOrganic chemistryKetoneStructural formula
The invention relates to a 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1H-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and a method for synthesizing the same. The structural formula of the compound is shown as a right formula. The method comprises the following steps that: trifluoro gamma-keto acid methyl ester and toluene sulfonic acid are dissolved in toluene and added with anhydrous magnesium sulfate in catalyst amount, and the mixture is subjected to refluxing reaction for 20 to 60 minutes in stirring, and then added with fluorine o-phenylendiamine; the mixture is continuously reacted for 10 to 15 hours and then added with toluene sulfonic acid in catalyst amount; the mixture is subjected to refluxing reaction for 10 to 15 hours; after the reaction, the mixture is separated and purified to obtain a white solid which is the 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1H-benzo[d]pyrrole[1,2-a]imidazole-1-ketone; and the molar ratio of the trifluoro gamma-keto acid methyl ester to the fluorine o-phenylendiamine is 1-1.2:1. The 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1H-benzo[d]pyrrole[1,2-a]imidazole-1-ketone has stronger activity and is absorbed more easily. Moreover, the method has the advantages of easily bought raw materials, quite simple operation, synthesis by a one-pot method, 58 percent of yield and suitability for mass production.
Owner:SHANGHAI UNIV
Synthesis method of 2,3-benzopyrrole compound NPS-1577
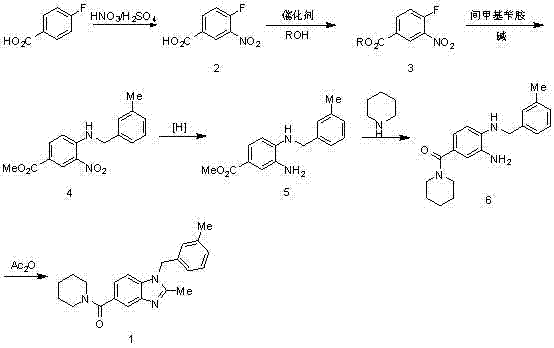
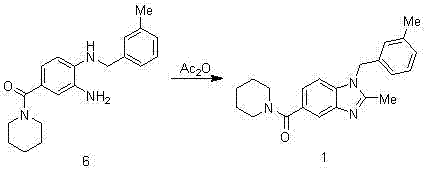
InactiveCN103113306AShort reaction cycleMild reaction conditionsOrganic chemistryBenzoic acidAcetic anhydride
The invention belongs to the technical field of pharmaceutical synthesis and particularly relates to a synthesis method of a 2,3-benzopyrrole compound NPS-1577. The method comprises the following steps of: (a) by taking 4-fluorobenzoic acid (2) as a raw material, nitrifying to prepare a compound; (b) carrying out esterification reaction by using thionyl chloride to obtain a compound 3; (c) reacting the compound 3 with 3-methylbenzylamine to a reaction to obtain an intermediate (4); (d) reducing the intermediate (4) by using hydrazine hydrate to obtain an intermediate (5); (e) carrying out aminolysis reaction on intermediate (5) and piperidine to obtain a compound (6); and (f) carrying out cyclization by virtue of acetic anhydride to obtain a final product (1). Compared with the existing synthesis method, the synthesis method provided by the invention is realized through six-step reactions and has the advantages of mild reaction condition for each-step reaction, high yield, cheap and available raw materials and short reaction period, and is simple and convenient to operate, so as to be very easy for large-scale industrial production.
Owner:TONGJI UNIV
3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof
InactiveCN101544657ARaw materials are easy to getEasy to operateOrganic chemistrySynthesis methodsKetone
The invention relates to 3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and a synthesis method thereof. The structural formula of the compound is shown as right. The method comprises the following steps: dissolving trifluoro gamma-methyl keto ester and catalytic dosage of paratoluenesulfonic acid into toluene, adding catalytic dosage of anhydrous magnesium sulfate, carrying out reflux reaction on the mixture for 20 to 60 minutes with stirring, and then adding ortho-aminophenol; adding the catalytic dosage of paratoluenesulfonic acid after reacting for 10 to 15 hours, performing reflux reaction for 10 to 15 hours, and ending the reaction; and performing separation and purification to obtain a white solid, namely 3'-trifluoromethyl-1-benzopyrrole ketopyrrolidine derivative. The 3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone creatively introduces a fluoridebearing group of trifluoromethyl with electron absorbing function to 3a position of benzopyrrole ketopyrrolidine so as to enhance the activity and be favorable for absorbing. In the invention, raw materials are easy to obtain, the operation is simple, the product is synthesized by a one-pot method, the productivity is up to 71 percent, and the product is suitable to be produced on a large scale.
Owner:SHANGHAI UNIV
Deep-color near-infrared-transmitting polypropylene compound and preparation method thereof
Owner:KINGFA SCI & TECH CO LTD
A kind of hydroxyl radical ratiometric fluorescent probe and its preparation method and application
ActiveCN111072699BHigh selectivityHigh sensitivityGroup 3/13 element organic compoundsColor/spectral properties measurementsFluoProbesArtemisinins
The present invention is by rhodamine B (Rho) and N 2 O-type benzopyrrole boron complexes (Bobpy) were linked to construct a novel fluorescence resonance energy transfer (FRET) probe Rho‑Bob for ratiometric fluorescence detection and imaging of OH. Rho‑Bob exhibits environmental affinity / hydrophobicity sensitivity and excellent mitochondrial localization ability. Rho‑Bob has been successfully applied for ratiometric fluorescence imaging of intracellular OH. These OH can be produced by Fenton reaction or by intracellular drug activation. The Rho‑Bob probe has high selectivity and sensitivity to hydroxyl radicals, and the detection limit is as low as nanomolar level (680nM). The present invention uses Rho-Bob to observe for the first time the ·OH produced by artemisinin molecules in the mitochondria of cells, and to discover for the first time that endogenous hydroxyl radicals exist in the gastrointestinal tract (GI) of zebrafish under normal culture conditions. The present invention not only provides a practical probe for ·OH detection and imaging, but also provides an important idea for constructing novel ratiometric probes for other ROS.
Owner:GUANGZHOU UNIVERSITY OF CHINESE MEDICINE
Novel protein kinase inhibitor, preparation method and application thereof
ActiveCN112300106AHigh activityEnhanced inhibitory effectOrganic chemistryAntineoplastic agentsBenzopyroneStructural formula
The invention belongs to the technical field of medicinal chemistry. The invention discloses a novel protein kinase inhibitor, of which the structural formula is shown as a formula I, wherein, R1 represents hydroxyl, carboxyl, acylamino or hydrogen; R2 represents nitro, amino, acylamino, hydroxyl, sulfydryl, halogen or trifluoromethyl; R3 represents alkoxy or hydroxyl; ring A represents benzopyrone, 1H-benzo[d]imidazole, substituted hydroxyl 1H-benzo[d]imidazole, benzoxazole, 1H-pyrrolo[2, 3-b]pyridine, benzopyrrole, 2, 3-dihydrobenzo[b][1, 4]dioxin, or 1H-indazole; X represents an ester groupor an amide group; and ring B represents a benzene ring, a nitrogen-containing five-membered heterocyclic ring or a nitrogen-containing six-membered heterocyclic ring. According to the invention, a novel protein kinase inhibitor is obtained by utilizing a splicing principle, and an anti-tumor effect is achieved by inhibiting tumor cell proliferation and promoting tumor cell apoptosis.
Owner:XUZHOU MEDICAL UNIV
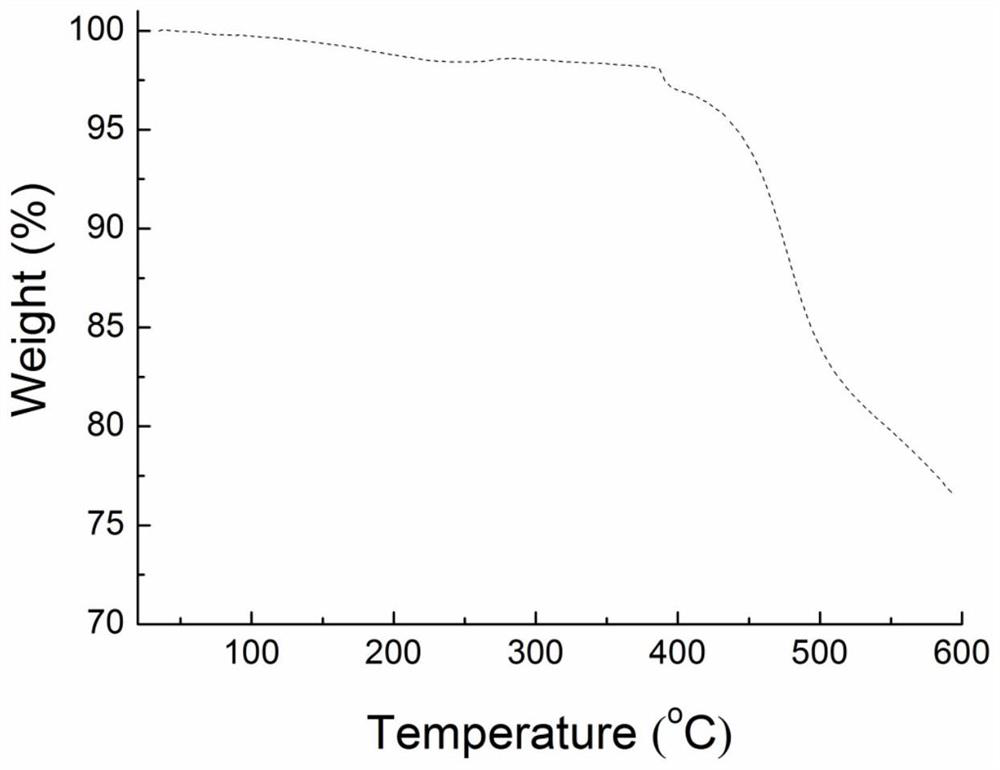
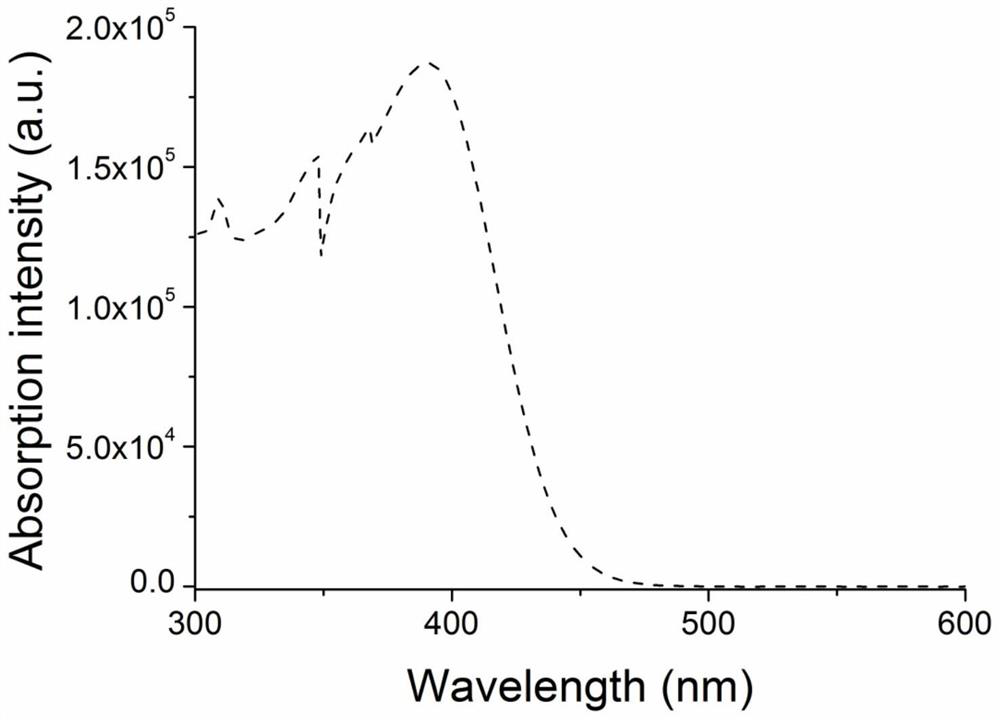
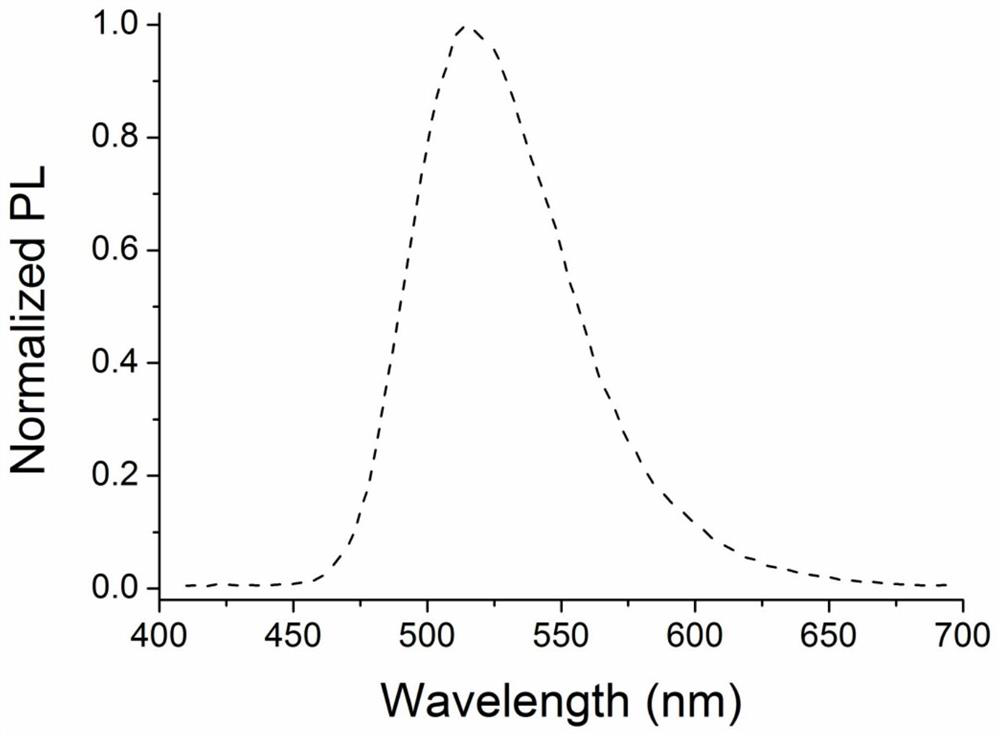
Novel organic electroluminescent material based on benzopyrroledione and application thereof
ActiveCN112707906AGood planarityImprove stabilityOrganic chemistrySolid-state devicesQuantum efficiencyDopant
The invention discloses a novel organic electroluminescent material based on benzopyrroledione and application thereof. Benzopyrroledione is used as a skeleton, and a type of fused ring molecules with a larger conjugate plane are constructed through cyclization and other series reactions, so that the non-radiative transition of the molecules is reduced, and the luminous efficiency of the material is improved; electron donating groups and electron withdrawing groups are further introduced into the molecules to regulate and control the photophysical properties of the material; and an organic light-emitting device is prepared through a solution method by taking the material as a light-emitting layer doping agent, and the maximum external quantum efficiency (2.2%) is obtained.
Owner:安徽秀朗新材料科技有限公司
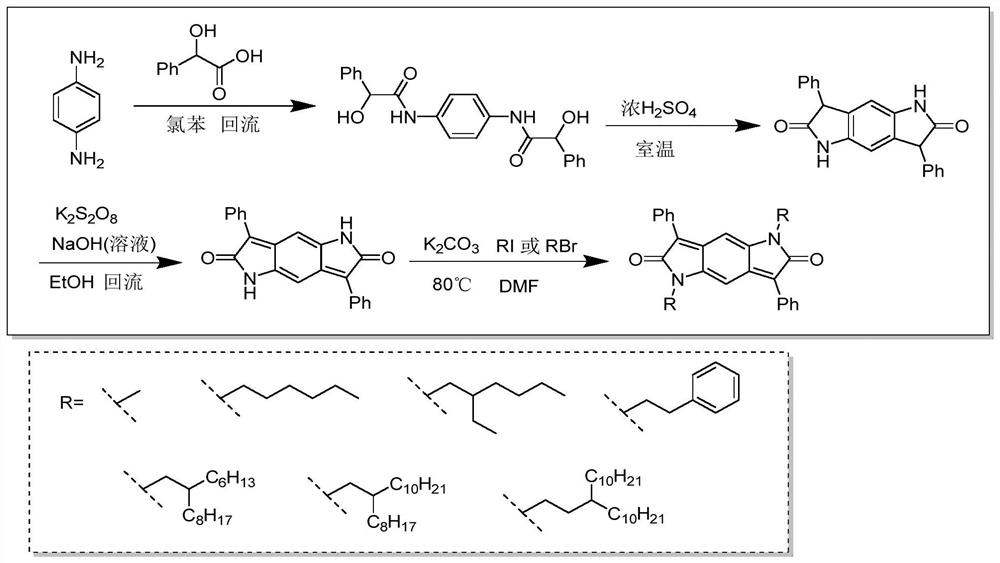
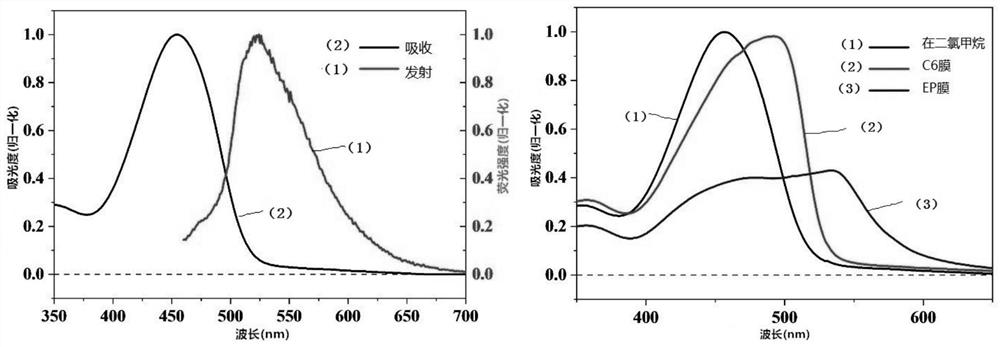
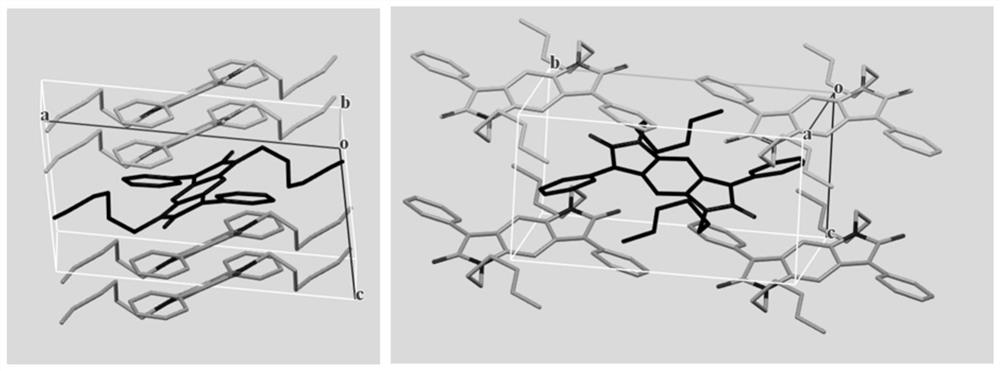
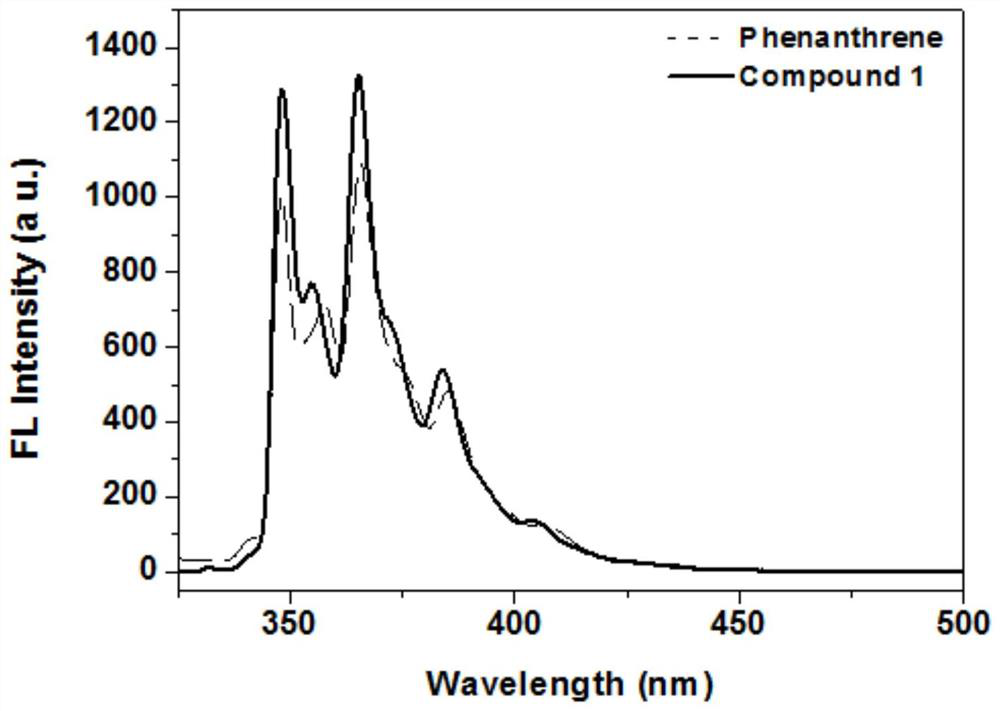
Synthesis method of intramolecular exciton splitting material with anti-aromaticity and quinonoid structure, preparation method of thin film and single crystal
ActiveCN113135919APolycrystalline material growthFrom normal temperature solutionsPhoto stabilitySingle crystal
The invention discloses a synthesis method of an intramolecular exciton splitting material with anti-aromaticity and a quinonoid structure and a preparation method of a thin film and a single crystal. The synthesis method of the intramolecular exciton splitting material comprises the following steps: synthesizing a molecule which takes benzopyrrolopyrroledione (BDPP) as a molecular skeleton and introduces different alkyl chain substituent groups on an amido bond nitrogen atom, wherein the skeleton has relatively strong blue-green light absorption, excellent air and light stability and appropriate triplet state energy. The invention discloses characterization means of the molecular solution and the thin film spectrum. The intramolecular exciton splitting material has an ultrafast-scale exciton splitting rate and a relatively high triplet state exciton yield, so that possibility is provided for application of a novel organic photovoltaic device to a great extent. The intramolecular exciton splitting (iSF) process in the BDPP molecule hardly depends on intermolecular orientation and electronic coupling, and the BDPP molecule has great potential to be integrated into an SF-based organic photovoltaic device.
Owner:CAPITAL NORMAL UNIVERSITY
(4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof
InactiveCN101544647ARaw materials are easy to getEasy to operateOrganic chemistryHexamethylenediamineKetone
The invention relates to (4aR,8aR)-3a-(difluoromethyl)decahydro-1H-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and a synthesis method thereof. The structural formula of the compound is shown as right. The method comprises the following steps: dissolving difluoro gamma-methyl keto ester and catalytic dosage of paratoluenesulfonic acid into toluene, adding catalytic dosage of anhydrous magnesium sulfate, stirring the mixture to reflux for 20 to 60 minutes, and then adding (1R,2R)-1,2- cyclohexanediamine; adding the catalytic dosage of paratoluenesulfonic acid after continuously reacting for 10 to 15 hours, wherein the mol ratio of the difluoro gamma-methyl keto ester to the (1R,2R)-1,2-cyclohexanediamine is (1-1.2):1; performing reflux reaction for 10 to 15 hours, and ending the reaction; and performing separation and purification to obtain a yellow sticky liquid, namely the (4aR,8aR)-3a-(difluoromethyl)decahydro-1H-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone. In the invention, raw materials are easy to obtain, the operation is simple, the product is synthesized by a one-pot method, the productivity is high up to 82 percent, and the product is suitable to be produced on a large scale.
Owner:SHANGHAI UNIV
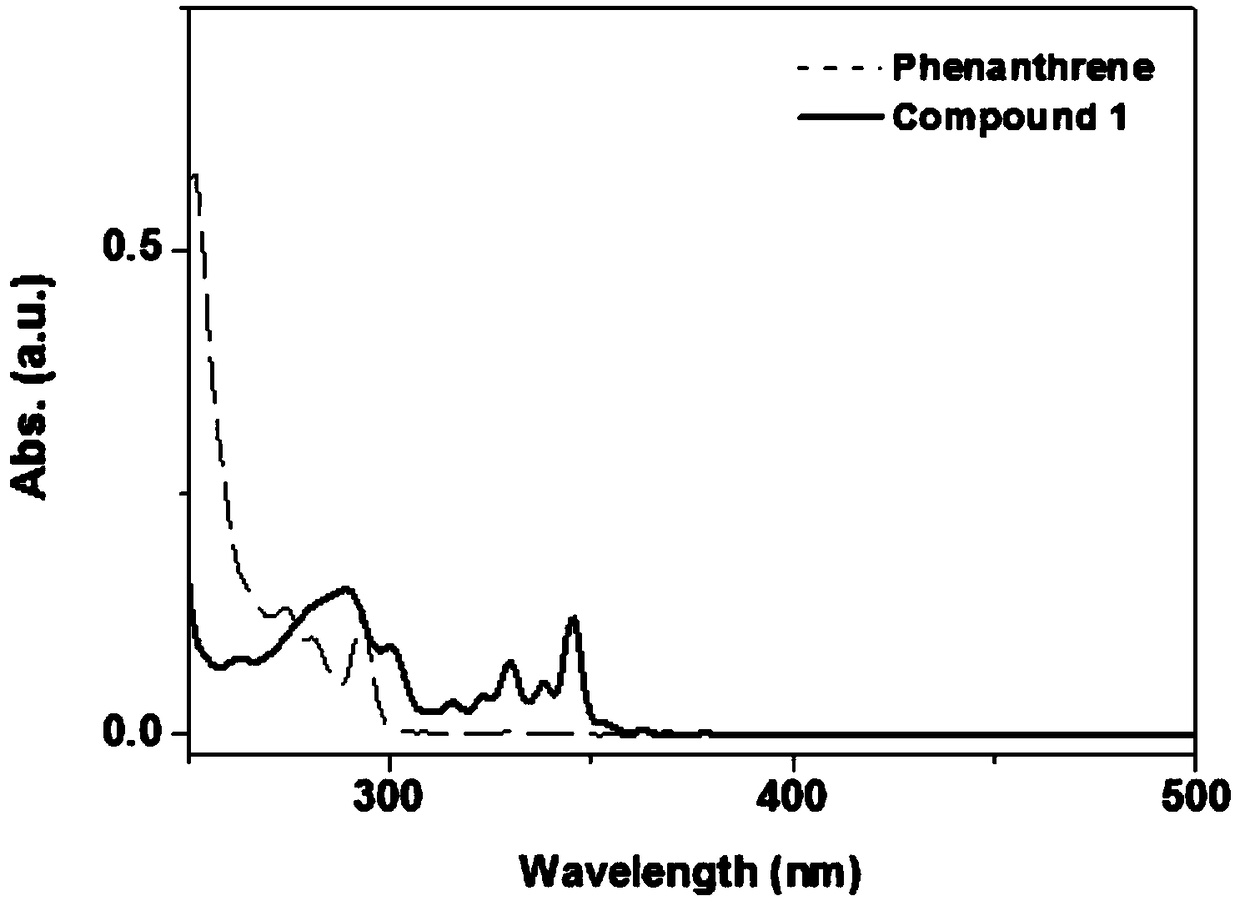
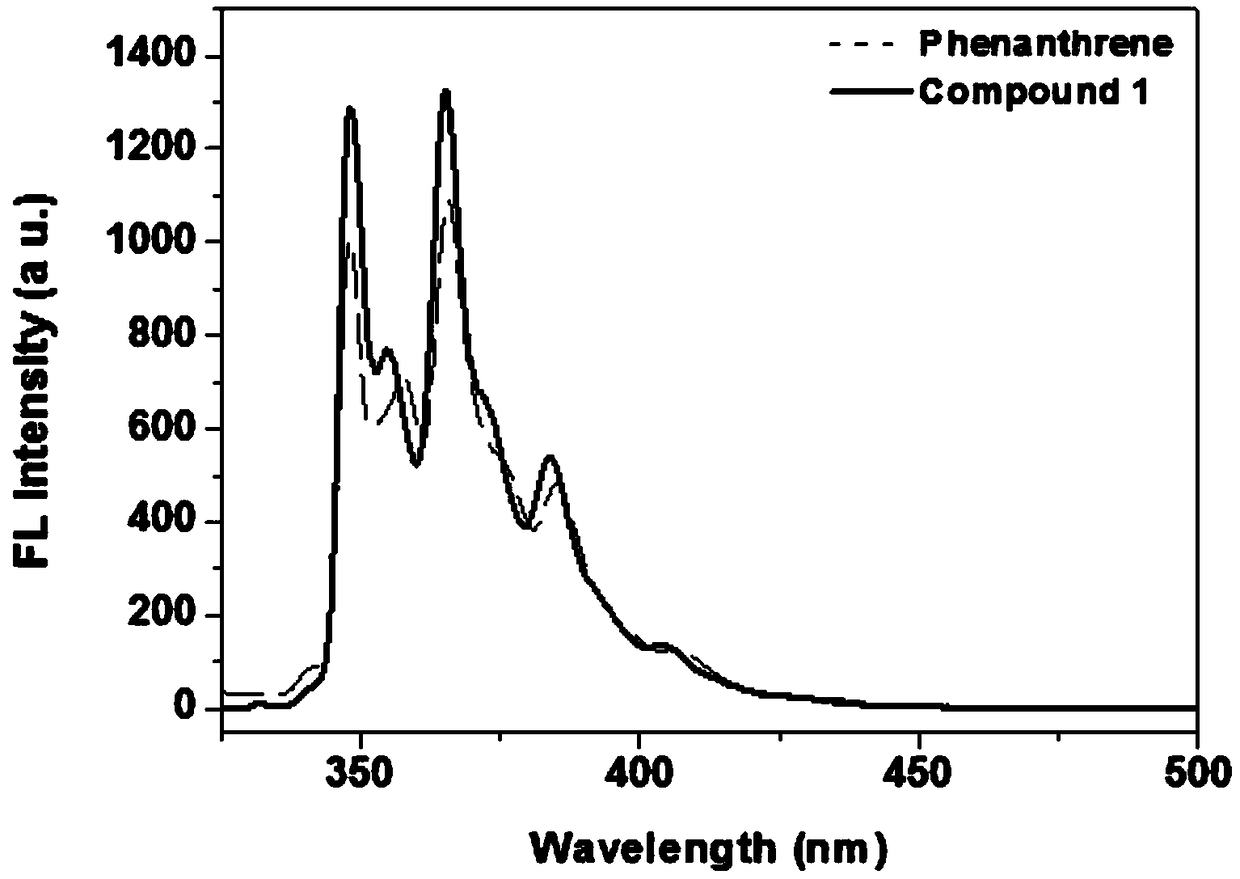
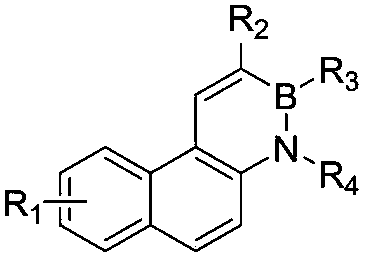
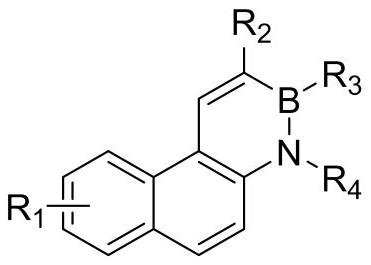
A kind of synthetic method of phenanthrene borazine and its derivatives
The present invention relates to a synthetic method of borazine phenanthrene and its derivatives, and tested the photoelectric physical properties of these compounds, and further studied the potential application value of this type of organic material in organic electrochemistry. The structural formula of this compound is: wherein R 1 , R 2 , R 3 , R 4 They are independently substituted or unsubstituted groups, including alkyl, aryl (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzopyridine, naphthalene ring , anthracycline, phenacene, tetracene, pyrene, linear or angled pentacene, hexacene, indene, fluorene, etc.). where R 1 , R 2 It can also be a single substituted halogen atom X: F, Cl, Br, I.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
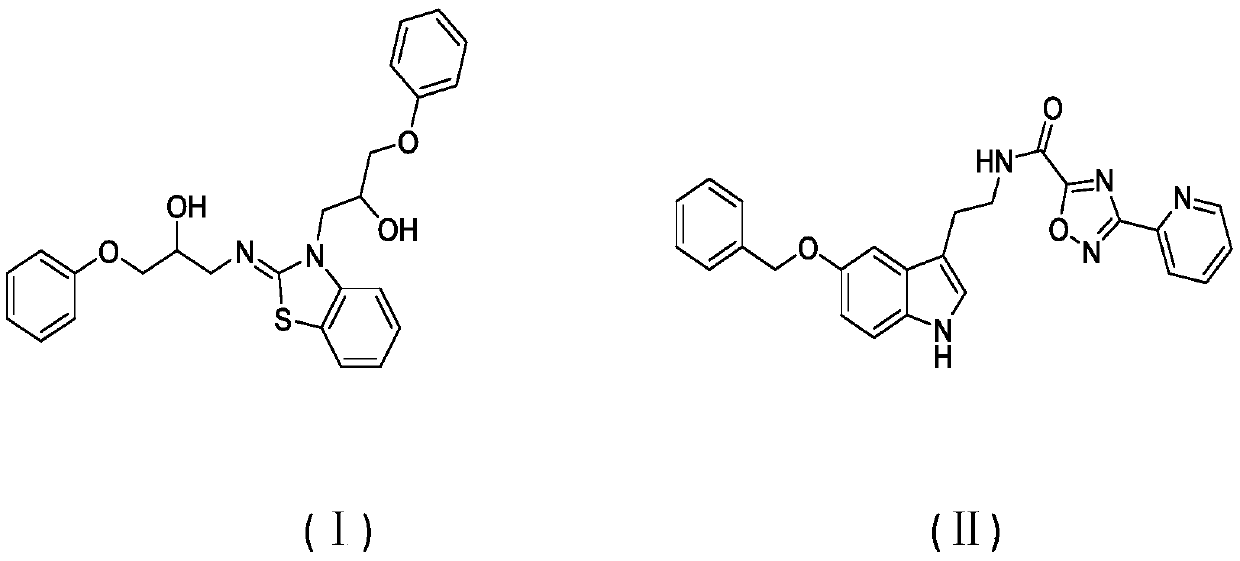
Substituted benzofuran, benzopyrrole, benzothiophene, and structurally related complement inhibitors
Disclosed are compounds of formulae I and II, and pharmaceutically acceptable salts and prodrugs thereof, which are inhibitors of the complement system. Also provided are pharmaceutical compositions comprising such a compound, and methods of using the compounds and compositions in the treatment or prevention of a disease or condition characterized by aberrant complement system activity.
Owner:BIOCRYST PHARM INC
Pyrrole-ring-containing boron-oxygen-doped polycyclic aromatic hydrocarbon as well as synthesis method and application thereof
PendingCN114276371AReduce usageThe synthesis method is simpleGroup 3/13 element organic compoundsFuranPyrazine
The invention relates to pyrrole ring-containing boron-doped polycyclic aromatic hydrocarbon as well as a synthesis method and application thereof, and tests of photoelectric physical properties and CV are carried out on the pyrrole ring-containing boron-doped polycyclic aromatic hydrocarbon so as to further research the potential application value of the organic material in the aspect of organic electrochemistry. The structural formula of the compound is as follows: Ra and Rb are respectively and independently hydrogen, deuterium, alkyl, alkoxy, naphthenic base, ether, heterocyclic group, phenyl, aryloxy, halogen, cyano or a combination of the hydrogen, deuterium, alkyl, alkoxy, naphthenic base, ether, heterocyclic group, phenyl, aryloxy, halogen and cyano; rc may be hydrogen, deuterium, alkyl, alkoxy, cycloalkyl, ether, heterocyclic aryl, phenyl, aryloxy, or a combination thereof; ar is a benzene ring, a thiophene ring, a furan ring, a pyrrole ring, a pyridine ring, benzothiophene, benzofuran, benzopyrrole, benzopyridine, a naphthalene ring, an anthracene ring, phenalene, carbazolyl, pyrazinyl, triphenyl, tetraphenyl, pyrene, linear or angular pentacene, hexacene, indene and fluorene; m is an integer of 0-5; and n is an integer of 0-3.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
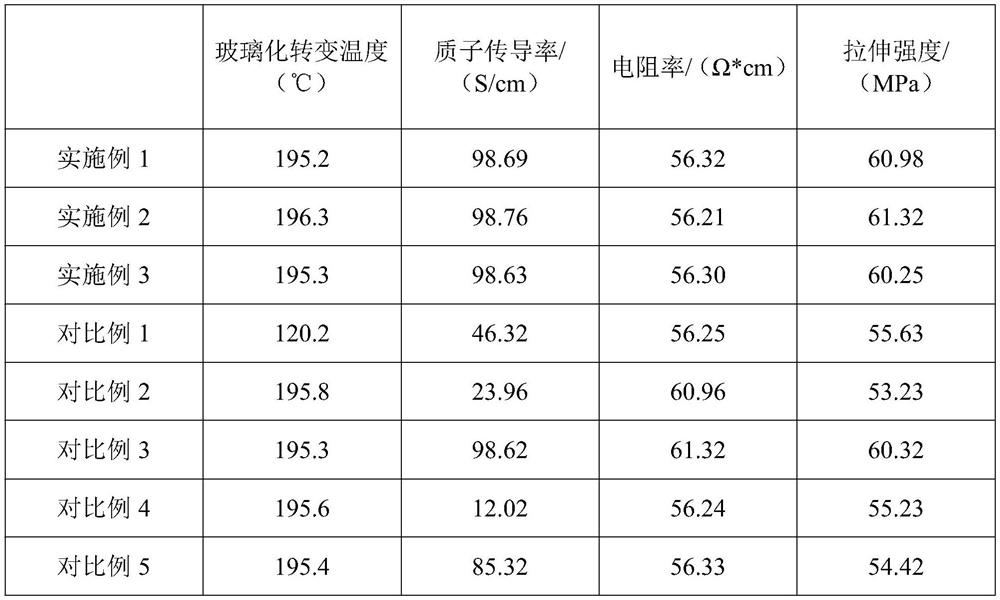
High-efficiency high-temperature-resistant proton conduction material and preparation method thereof
PendingCN114316290AImprove high temperature resistanceHigh porositySolid electrolyte fuel cellsPyrroleThioxanthene
The invention discloses a high-efficiency high-temperature-resistant proton conduction material and a preparation method thereof, and relates to the technical field of proton conduction materials. The preparation method comprises the following steps: firstly, mixing trimalonic acid (triphenylmethoxy) silane and 4, 5-diamino-o-chloro-sulfurized benzoyl chloride to form a thioxanthone microporous polymer, and preparing high-temperature-resistant nano gel particles; the preparation method comprises the following steps: preparing nano gel particles, mixing tri-o-aminobenzoquinonyl benzene, 2-sulfydryl-3-carbonyl-4-nitrobutanol and the nano gel particles to form a benzopyrrole compound, forming hyperbranched amide through a photosynthesis-assisted ultrasonic process, and stably embedding the nano gel particles in a cavity of the hyperbranched amide to obtain the hyperbranched polymer. And the density of sulfonate ions in the mass transfer channel is increased during use, so that the prepared high-efficiency high-temperature-resistant proton conduction material has good high temperature resistance, tensile strength, conductivity and proton conductivity.
Owner:沈金国
Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound
The invention discloses a synthesis method of a benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H) diketone compound, and belongs to the technical field of organic synthesis. According to the technical scheme, the preparation method is characterized by comprising the following steps: dissolving a 2-aryl indole compound 1 and an N-substituted maleimide compound in a solvent, adding a catalyst and an oxidant, and reacting at 80-120 DEG C to obtain the target product benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound. The target product benzo [a] pyrrolo [3, 4-c] carbazole-1, 3(2H, 8H)-diketone compound is prepared through [4 + 2] oxidative cyclization reaction between the 2-aryl indole compound and the N-substituted maleimide compound, and the method has the advantages ofsimplicity and convenience in operation, mild conditions, wide substrate application range and the like, and is suitable for industrial production.
Owner:HENAN NORMAL UNIV
Ulllazine derivatives containing diarylboron and diarylamine and synthesis method thereof
The invention relates to a plurality of Ulllazine derivatives containing diarylboron and diarylamine and a synthesis method thereof. The photoelectric material quantity properties of the compounds aretested, and the potential application values of the compounds in the aspect of organic photoelectricity are further studied. The structural formulas of the compounds are shown in the description, wherein R1 groups are independent and can be single substituted halogen atoms X: F, Cl, Br and I, or hydrogen, olefin, alkyne, alkyl, aldehyde group or ketone, or substituted or unsubstituted aryl and heteroaryl (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzopyridine, naphthalene ring, anthracene ring, phenalene, tetracene, pyrene, chrysene, linear or angled pentacene, hexacene, indene, fluorene and the like), or a diarylboron group or a diarylamine group, Ar1 and Ar2 represent different types of aryl groups (benzene ring, thiophene ring, furan ring, pyrrole, pyridine, benzothiophene, benzofuran, benzopyrrole, benzopyridine, naphthalene ring, anthracene ring, phenadiene, tetracene, pyrene, chrysene, linear or angled pentacene, hexacene, indene, fluorene and the like).
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound](https://images-eureka.patsnap.com/patent_img/101bbbb8-64cd-4dad-97da-7f8896dd0091/HDA0001035003560000011.png)
![A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound](https://images-eureka.patsnap.com/patent_img/101bbbb8-64cd-4dad-97da-7f8896dd0091/HDA0001035003560000012.png)
![A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound A kind of preparation method of 2-methyl-1,2,3,9-tetrahydrobenzo[b]pyrrole[1,4]-thiazine-1,3-dione compound](https://images-eureka.patsnap.com/patent_img/101bbbb8-64cd-4dad-97da-7f8896dd0091/GDA0001451398840000011.png)
![Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same](https://images-eureka.patsnap.com/patent_img/1874cc91-c4a0-4254-b395-3553d262735a/HDA0002461657850000011.png)
![Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same](https://images-eureka.patsnap.com/patent_img/1874cc91-c4a0-4254-b395-3553d262735a/HDA0002461657850000012.png)
![Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same Benzo[c]pyrrolidone copolycarbonate optical articles, articles formed therefrom, and methods of making the same](https://images-eureka.patsnap.com/patent_img/1874cc91-c4a0-4254-b395-3553d262735a/BDA0002461657840000021.png)
![6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same](https://images-eureka.patsnap.com/patent_img/29dfc056-5d68-4fff-90c9-64b31931bce8/a200910048343c00021.PNG)
![6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same](https://images-eureka.patsnap.com/patent_img/29dfc056-5d68-4fff-90c9-64b31931bce8/a200910048343d00031.PNG)
![6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same 6-flourine-3a-(trifluoromethyl)-2,3,3a,4-tetrahydro-1h-benzo[d]pyrrole[1,2-a]imidazole-1-ketone and method for synthesizing same](https://images-eureka.patsnap.com/patent_img/29dfc056-5d68-4fff-90c9-64b31931bce8/a200910048343d00041.PNG)
![3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof 3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/3222edc1-5de5-4b3b-9350-14ce7e6db132/a200910048352c00021.PNG)
![3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof 3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/3222edc1-5de5-4b3b-9350-14ce7e6db132/a200910048352d00041.PNG)
![3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof 3a-(trifluoromethyl)-3,3a-dihydrobenzene[d]pyrrole[2,1-b]oxazole-1(2H)-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/3222edc1-5de5-4b3b-9350-14ce7e6db132/a200910048352d00042.PNG)


![(4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof (4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/54eca619-7326-441d-ac09-b7ead04e5910/a200910048349c00021.PNG)
![(4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof (4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/54eca619-7326-441d-ac09-b7ead04e5910/a200910048349d00031.PNG)
![(4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof (4ar,8ar)-3a-(difluoromethyl)decahydro-1h-benzene[d]pyrrole[1,2-a]-thiazole-1-ketone and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/54eca619-7326-441d-ac09-b7ead04e5910/a200910048349d00041.PNG)
![Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound](https://images-eureka.patsnap.com/patent_img/c5c71a80-23b9-4829-9ec5-48bbf69cbb4d/FDA0002746295550000011.png)
![Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound](https://images-eureka.patsnap.com/patent_img/c5c71a80-23b9-4829-9ec5-48bbf69cbb4d/BDA0002746295560000021.png)
![Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound Synthesis method of benzo [a] pyrrolo [3, 4-c] carbazole-1, 3 (2H, 8H)-diketone compound](https://images-eureka.patsnap.com/patent_img/c5c71a80-23b9-4829-9ec5-48bbf69cbb4d/BDA0002746295560000022.png)