Novel protein kinase inhibitor, preparation method and application thereof
A protein kinase inhibitor, a new technology, used in organic chemistry, anti-tumor drugs, drug combinations, etc., can solve the problems of weak effect, underdevelopment, acquired drug resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
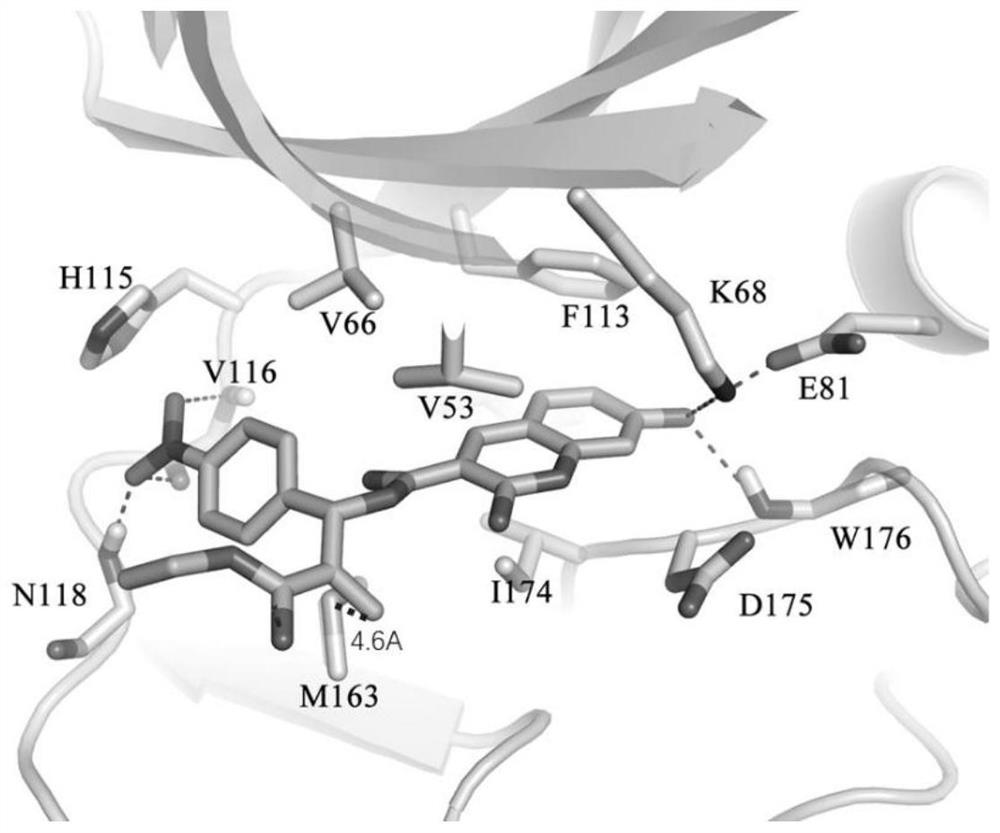
Image
Examples
Embodiment 1
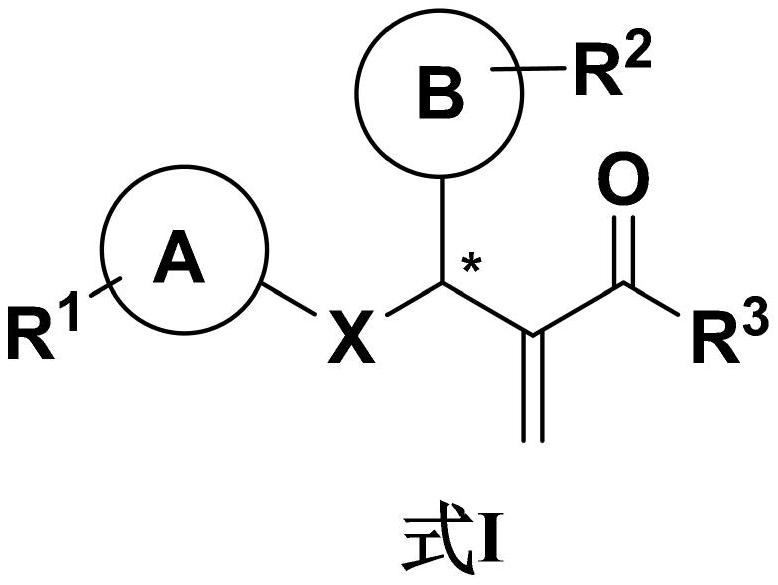
[0037] In view of the many types of protein kinase inhibitors prepared in the present invention, the applicant only lists one class of compounds, and other compounds can achieve the following technical effects. The compound of formula I-1 is a class of compounds of formula I, and its structural formula is as follows:
[0038]
[0039] The concrete steps of preparation formula Ⅰ-1 are as follows:
[0040]
[0041] I is a compound of XI, and II is a compound of formula II.
[0042] R 1 Represents hydrogen, halogen, nitro, methoxy, trifluoromethyl, methoxy, methyl;
[0043] R 2 represents a hydrocarbon group;
[0044] R 3 : hydroxyl, hydrogen.
[0045] The specific preparation method is: dissolve II (compound of formula II) (1 equivalent) in thionyl chloride (1.5mL, 20mmol), heat up to 80°C for 4h, concentrate to remove excess thionyl chloride, and dry with toluene , add I(XI compound) (1 equivalent), add triethylamine (1 equivalent), stir at room temperature for 48h,...
Embodiment 2
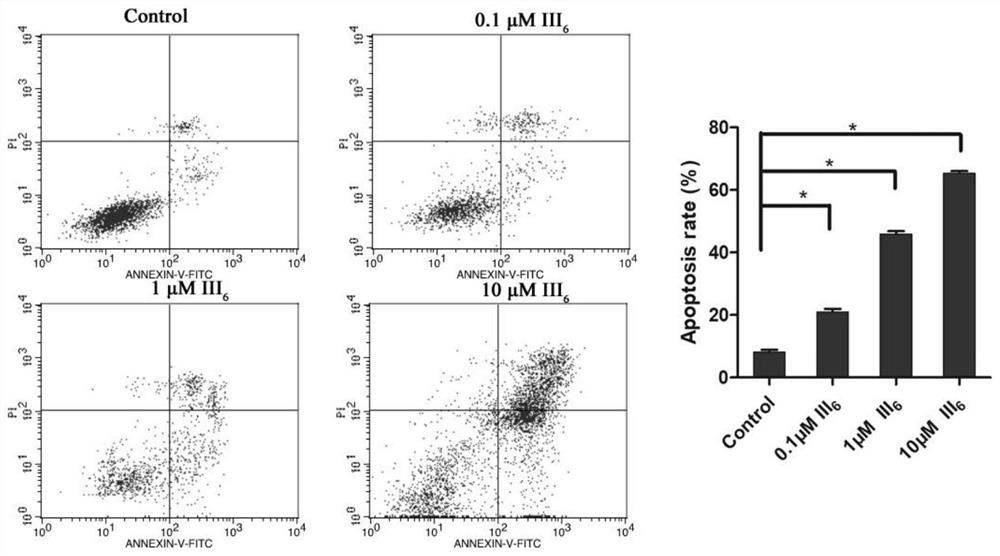
[0054] CCK8 method was used to determine the inhibitory effect of compounds on renal cancer cell line ACHN, breast cancer cell line MCF7, colorectal cancer cell line HCT116 and liver cancer cell line HepG2. Add 100 μL (about 5000 cells) of tumor cell suspension in logarithmic growth phase to each well of a 96-well plate, and culture the plate in an incubator for 24 hours (37°C, 5% CO 2 ), add 100 μL of the test compound with a concentration of 10 μmol / L to each well, incubate in the incubator for 72 hours or 96 hours, discard the liquid in the well, add 10 μL of CCK-8 solution to each well in the dark and incubate in the incubator for 0.5- After 4 hours, the absorbance was measured with a microplate reader at a wavelength of 450-570 nm, and the inhibitory rate of the test compound against each tumor cell at a single concentration (10 μmol / L) was calculated.
[0055] Table 1 Effect of 10μM compounds on tumor cell proliferation inhibition
[0056]
[0057]
[0058] Known ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com