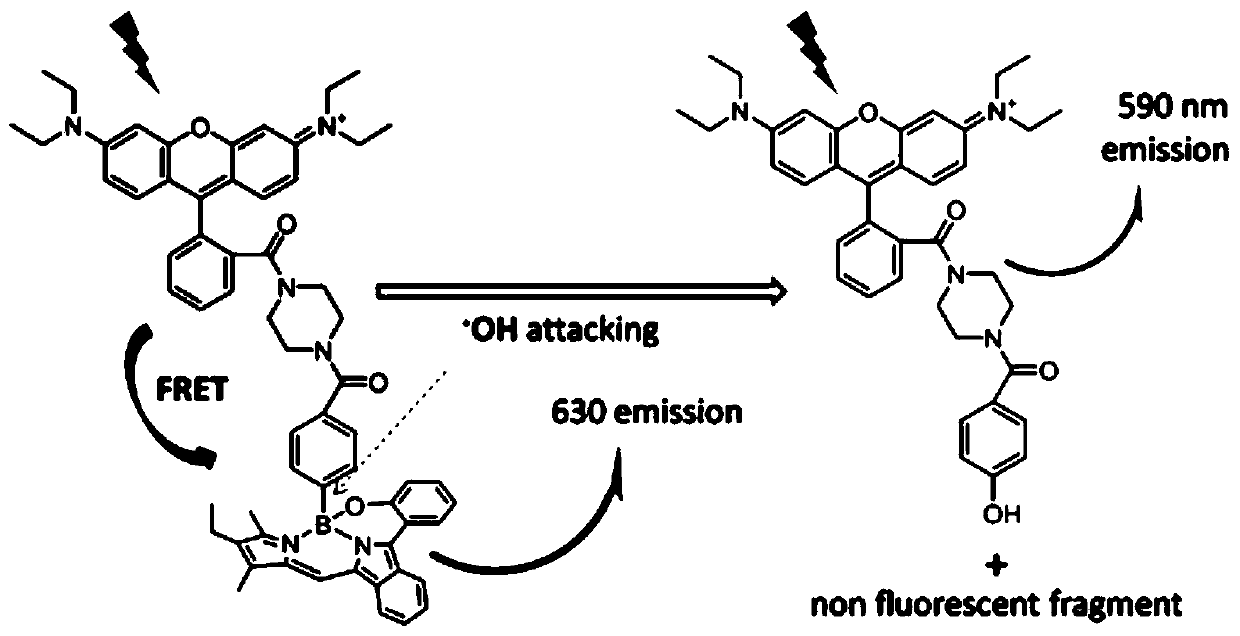
Hydroxyl radical ratio type fluorescent probe as well as preparation method and application thereof
A technology of fluorescent probes and free radicals, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of limited applications, achieve high selectivity and sensitivity, and excellent mitochondrial localization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Synthesis of embodiment 1 fluorescent probe
[0061] The synthetic route of the fluorescent probe is as follows:
[0062]
[0063]
[0064] Wherein, above-mentioned reaction condition is: a.POBr 3 , DMF / DCM, 45°C; b.Pd(PPh 3 ) 4 , Na 2 CO 3 , toluene, 75°C, EtOH / NaOH reflux; c. 4-dimethyl-3-ethylpyrrole, POCl 3 , DCM, 0° C., then 4-carboxyphenylboronic acid, DCM, reflux; d. EDC, HOBt, DMF, room temperature.
[0065] (1) Compound 3 was synthesized according to the reported method
[0066]
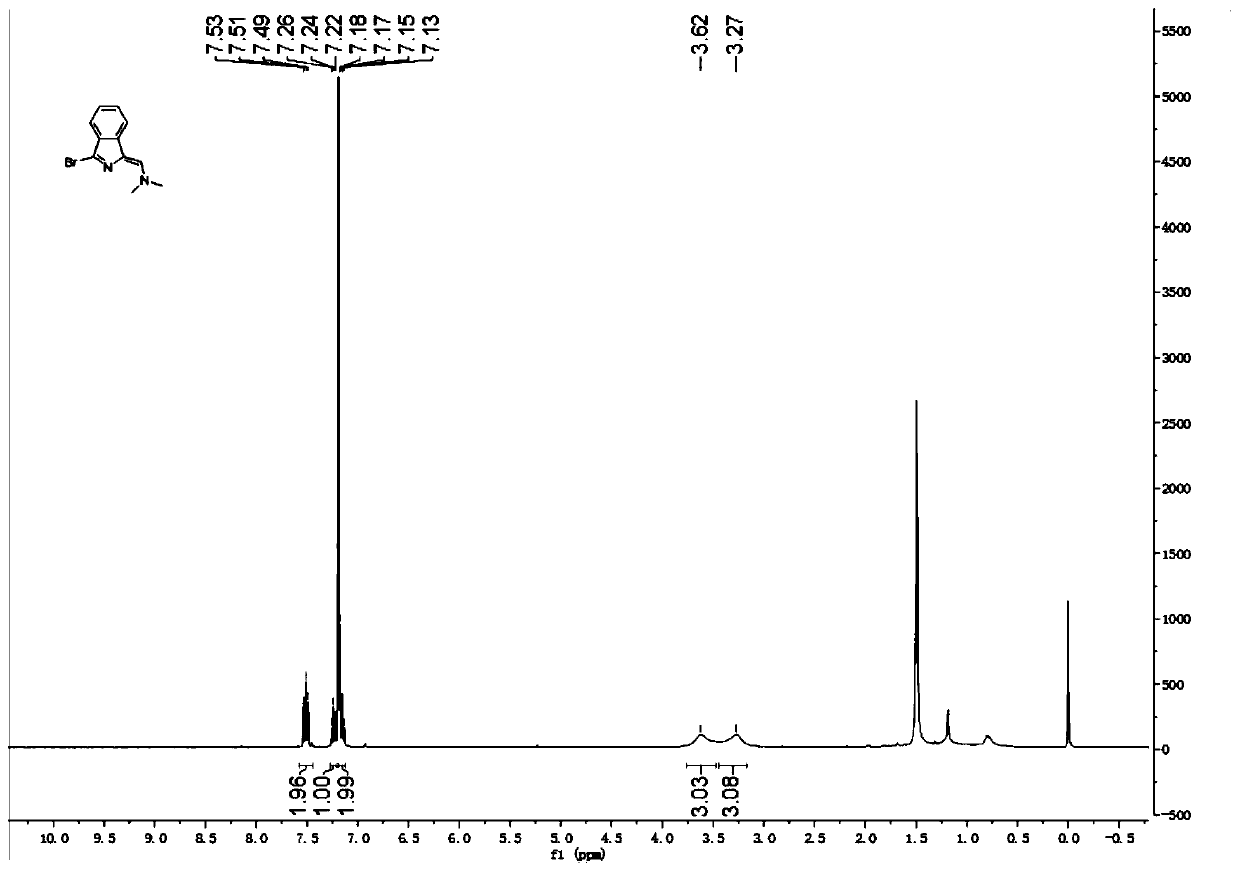
[0067] Compound 2: black solid (84%). 1 H NMR (400MHz, CDCl 3 )δ7.53–7.49(m,2H),7.26–7.22(m,1H),7.18–7.13(m,2H),3.62(s,3H),3.27(s,3H).ESI[M+H] + m / z calcd.for[C 11 h 12 BrN 2 ]251.01,found 251.00[M+H] + .Compound 2 1 H NMR spectrum as figure 2 shown.
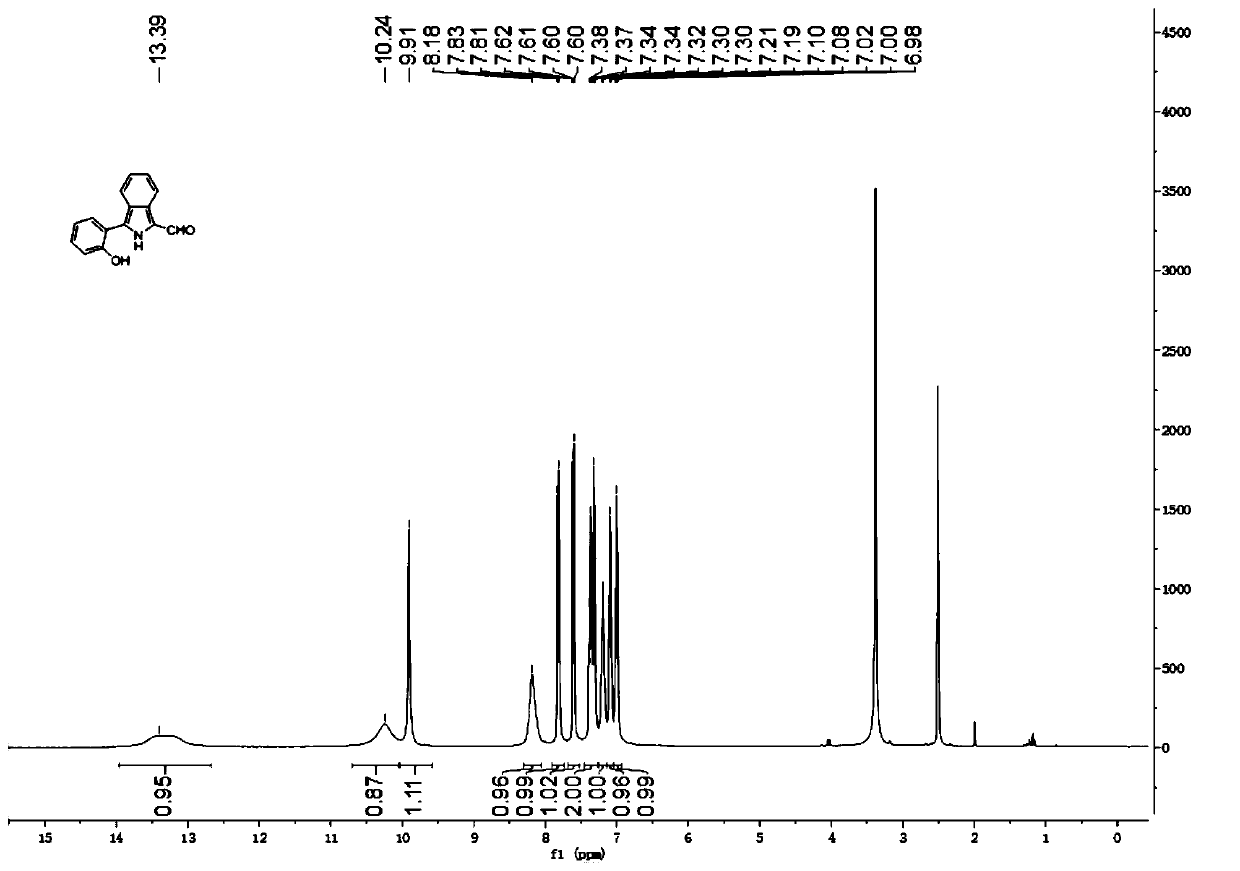
[0068] Compound 3: yellow solid (75%). 1 H NMR (400MHz, d 6 -DMSO)δ13.39(s,1H),10.24(s,1H),9.91(s,1H),8.18(s,1H),7.82(d,J=8.4Hz,1H),7.62–7.60(m ,1H),7.38–7.30(m,2H),7.21–7.19(s,1H),7.09(d,J=8.0Hz,1H),7.02–6....
Embodiment 2
[0080] The photophysical property of embodiment 2 compound 4
[0081] The photophysical properties of compound 4 were tested, and the test results were as follows Figure 11 shown. Among them, A is ACN / H 2 O is the fluorescence spectrum of compound 4 (20 μM) in mixed solution; B is the fluorescence spectrum of compound 4 (20 μM) in ACN / PBS 2:1 solution before and after OH reaction, excited at 580 nm; C is before and after OH reaction, The UV-vis absorption spectrum of corresponding compound 4, OH is produced by the following conditions (H 2 o 2 50 μM, EDTA-Fe 2+ 200 μM). Figure 11 The fluorescence spectrum in A shows that the fluorescence intensity of compound 4 is highly sensitive to solvent hydrophobicity / hydrophilicity changes. Compound 4 has weak fluorescence in pure water, but in acetonitrile (ACN) / H 2 The emission intensity in mixed solvents of O varies with ACN and H 2 O volume ratio increases. This feature provides a potential possibility for the probe's memb...
Embodiment 3
[0090] Example 3 Reaction Kinetics of Compound 4 and OH
[0091] Coumarin-3-carboxylic acid (Coumarin-CA) is a commercial fluorescent probe for ·OH detection. The present invention selects Coumarin-CA and compound 4 for comparison. • The reaction between OH and Coumarin-CA produces the fluorescent product 7-hydroxycoumarin-3-carboxylic acid with an emission peak at 454 nm under excitation at 400 nm. Therefore, check the fluorescence intensity I 454 The reaction can then be monitored. Meanwhile, the reaction of OH with compound 4 can reduce its absorbance at 610 nm, thus providing a simple method to monitor the reaction. When testing, Coumarin-CA (100 μM) or compound 4 (40 μM) was mixed with Fenton’s reagent EDTA-Fe2 + (100μM) / H 2 o 2 (10 mM) in sodium borate buffer (pH 9.0). In the competition experiment, Coumarin-CA and compound 4 were mixed in advance, and then reacted with ·OH. The reaction was carried out in a 96-well plate, and the emission intensity I under excit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com