Synthesis method of diboron azatriphenylene and derivative thereof
A synthesis method and a derivative technology, which are applied in the field of synthesis of bisboraphenanthroline and its derivatives, can solve the problems that potential application characteristics have not been widely developed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
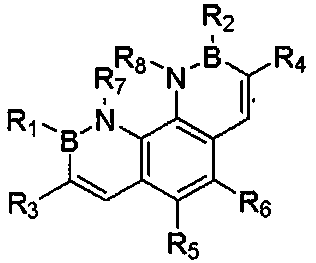
[0026] A kind of overall synthetic method of borazine phenanthrene and its derivatives of the present invention comprises the following synthetic routes and steps:
[0027]
[0028] Some of the above-mentioned compounds are given examples, and the details are as follows:
Embodiment 1
[0029] Example 1: Synthesis of 2,9-diphenyl-1,2,9,10-azaboraphenanthrene compound 3
[0030] 1) Synthesis of compound 2: Weigh 3,6-dibromo-1,2 phenylenediamine (1.0equiv, 22.8mmol, 6g), tetrakistriphenylphosphine palladium (0.02equiv, 0.456mmol, 524mg) and pump gas Three times, under nitrogen protection, tri-n-butylvinyltin (3.0 equiv, 68.4 mmol, 21.7 g) was added and anhydrous toluene was added to reflux at 110° C. for 24 h. After the reaction was complete, it was filtered, spin-dried toluene, and quickly analyzed by column chromatography to obtain the yellow target compound.
[0031] 2) Synthesis of compound 3a: Add 3,6-divinyl-1,2-phenylenediamine (1.0equiv, 1.87mmol, 300mg) into a 100mL flask, add anhydrous toluene under nitrogen protection, and place at -30°C Add (3.0equiv, 5.6mmol5.6mL) boron trichloride, reflux for 24 hours, remove the solvent after the reaction, add anhydrous diethyl ether, add (3.0equiv, 1.87 mmol, 71mg) lithium aluminum hydride at -30°C, Stir at ro...
Embodiment 2
[0033] Example 2: Synthesis of 2,9-diphenyl-1,2,9,10-azaboraphenanthrene derivatives
[0034] 4) Synthesis of compound 4-2Br: Weigh 2,9-diphenyl-1,2,9,10-azaboraphenanthrene (1.0equiv, 0.142mmol, 46mg), add dichloromethane to dissolve it completely Stir at 0°C for 15min, add 1mol / L liquid bromine-dichloromethane solution (2.1equiv, 0.299mmol, 299ul), stir for 15min, take it out and stir at room temperature for 0.5h, after the reaction is complete, add water and dichloromethane to extract , dried with anhydrous magnesium sulfate, filtered, spin-dried, and separated by column chromatography to obtain the white target compound.
[0035] 5) Synthesis of compound 4-4Br: Weigh 2,9-diphenyl-1,2,9,10-azaboraphenanthrene (1.0equiv, 0.210mmol, 70mg), add dichloromethane to dissolve it completely Stir at 0°C for 15min, add 1mol / L liquid bromine-dichloromethane solution (6.0equiv, 0.1.264mmol, 1.264ml), stir for 15min, take it out and stir at room temperature for 12h, when the reaction i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com