Pyrrole-ring-containing boron-oxygen-doped polycyclic aromatic hydrocarbon as well as synthesis method and application thereof
A technology of fused aromatic hydrocarbons and pyrrole rings, which is applied in the field of boron-containing polycyclic aromatic heterocyclic organic compounds and their synthesis, can solve the problems of lack of synthesis methods and instability, and achieve the effect of simple and easy synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
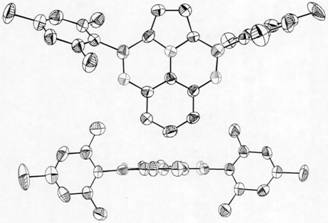
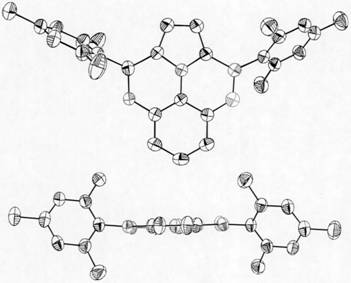
Image
Examples
Embodiment 1
[0081]
[0082] Formula 13 The synthetic route of the single BO-doped compound containing pyrrole ring
[0083] Synthesis of Compound 2: Weigh 2-methoxyaniline (218mg, 1.77mmol, 1.0equiv) and dissolve it in 5.00mL of glacial acetic acid, add 2,5-dimethoxytetrahydrofuran (0.23mL, 1.77mmol, 1.0equiv) . The reaction was stirred at 80°C for 4 hours. After cooling down to room temperature, it was extracted with dichloromethane and saturated aqueous sodium bicarbonate solution, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, followed by column chromatography (eluent was petroleum ether:ethyl acetate=20:1) to obtain colorless liquid 2 (155mg, 51%).
[0084] 1 H NMR (400MHz, CDCl 3 ): δ7.27-7.35 (m, 2H, Ar), 7.00-7.08 (m, 4H, Ar), 6.35 (t, J=2.0 Hz, 2H, Ar), 3.86 (s, 3H, OCH 3 ).
[0085] Synthesis of compound 3b: under the protection of an inert gas atmosphere, weigh 1-(2-methoxyphenyl)-1H pyrrole (247mg, 1.43mmol, 1.00equ...
Embodiment 2
[0088]
[0089] The synthetic route of the double BO-doped compound of formula 14 containing pyrrole ring
[0090] Synthesis of Compound 5: Weigh 2,6-dimethoxyaniline (1.03g, 6.71mmol, 1.00equiv)) and dissolve it in glacial acetic acid (0.47mL), 1,2-dichloroethane (12.48mL) and water (7.52mL) in a mixed solvent, when the temperature was heated to 80°C, 2,5-dimethoxytetrahydrofuran (0.90mL, 7.05mmol, 1.05equiv) was added, and the reaction was stirred at 80°C for 11 hours, and then cooled to room temperature , extracted with dichloromethane and saturated brine, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, followed by column chromatography (eluent: petroleum ether: ethyl acetate = 10:1) to obtain a white solid 5 ( 978 mg, 69%).
[0091] 1 H NMR (400MHz, CDCl3): δ7.28 (t, J=8.0Hz, 1H, Ar), 6.77 (t, J=2.0Hz, 2H, Ar), 6.67 (d, J=8.4Hz, 2H, Ar ), 6.33(t, J=2.0Hz, 2H, Ar), 3.78(s, 6H, OCH 3 ).
[0092] Synthesis of compoun...
Embodiment 3
[0095]
[0096] Formula 15 The synthetic route of the BN-BO doped compound containing pyrrole ring
[0097] Synthesis of compound 8: Weigh 2-methoxy 6-nitroaniline (522mg, 3.11mmol, 1.0equiv) and dissolve it in 20.00mL glacial acetic acid, add 2,5-dimethoxytetrahydrofuran (0.40mL, 3.11mmol , 1.0 equiv). The reaction was heated under reflux at 123°C for 2 hours, and after cooling down to room temperature, it was extracted with dichloromethane and saturated aqueous sodium bicarbonate, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed under reduced pressure, followed by column chromatography (eluent (petroleum ether: ethyl acetate = 10:1) to obtain 8 (554 mg, 82%) as a yellow solid.
[0098] 1 H NMR (400MHz, CDCl 3 ): δ7.47 (dd, J 1 =8.4Hz,J 2 =8.4Hz, 1H, Ar), 7.40(dd, J 1 = 1.2Hz, J 2 =8.4Hz, 1H, Ar), 7.25(dd, J 1 = 1.2Hz,J 2 =8.4Hz, 1H, Ar), 6.73(t, J=2.4Hz, 2H, Ar), 6.36(t, J=2.0Hz, 2H, Ar), 3.87(s, 3H, OCH 3 ).
[0099] Synthesis of C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com