Synthetic method of bis-pyrrole fused boron naphthazine and derivatives of bis-pyrrole fused boron naphthazine
A synthesis method and technology of bispyrrole, applied in the field of synthesis of bispyrrole-fused borazanthene and its derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
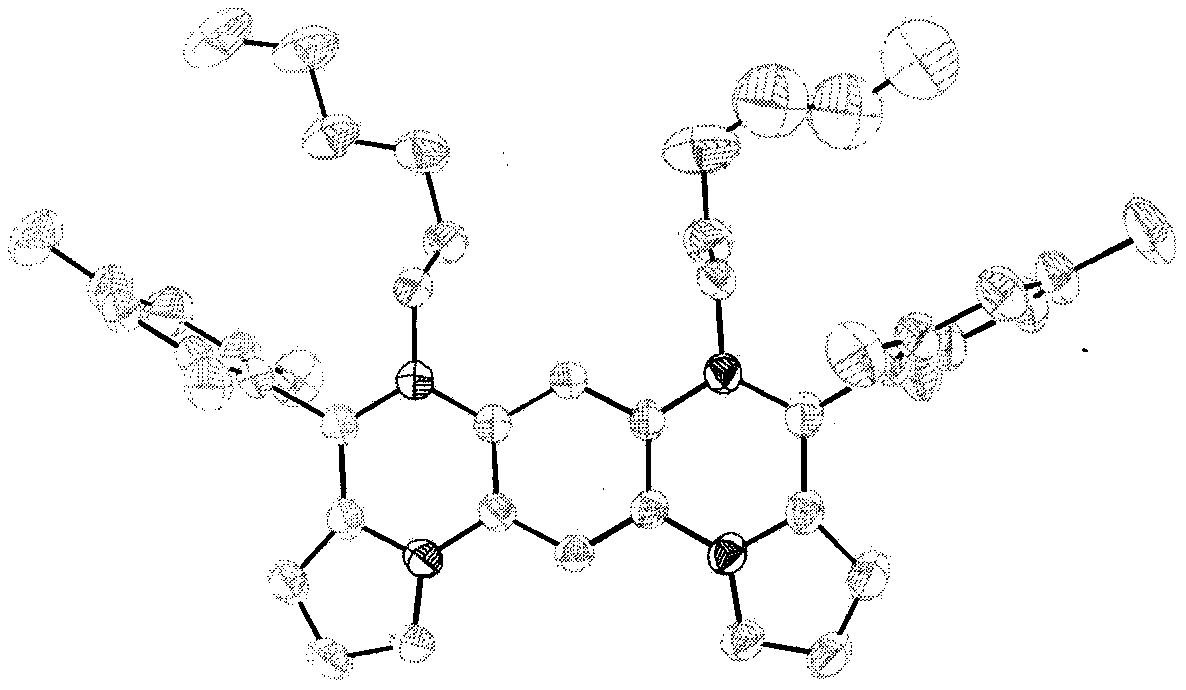
[0032] An overall synthesis method of bispyrrole-fused borazanthene and derivatives thereof of the present invention comprises the following synthesis route and steps:
[0033]
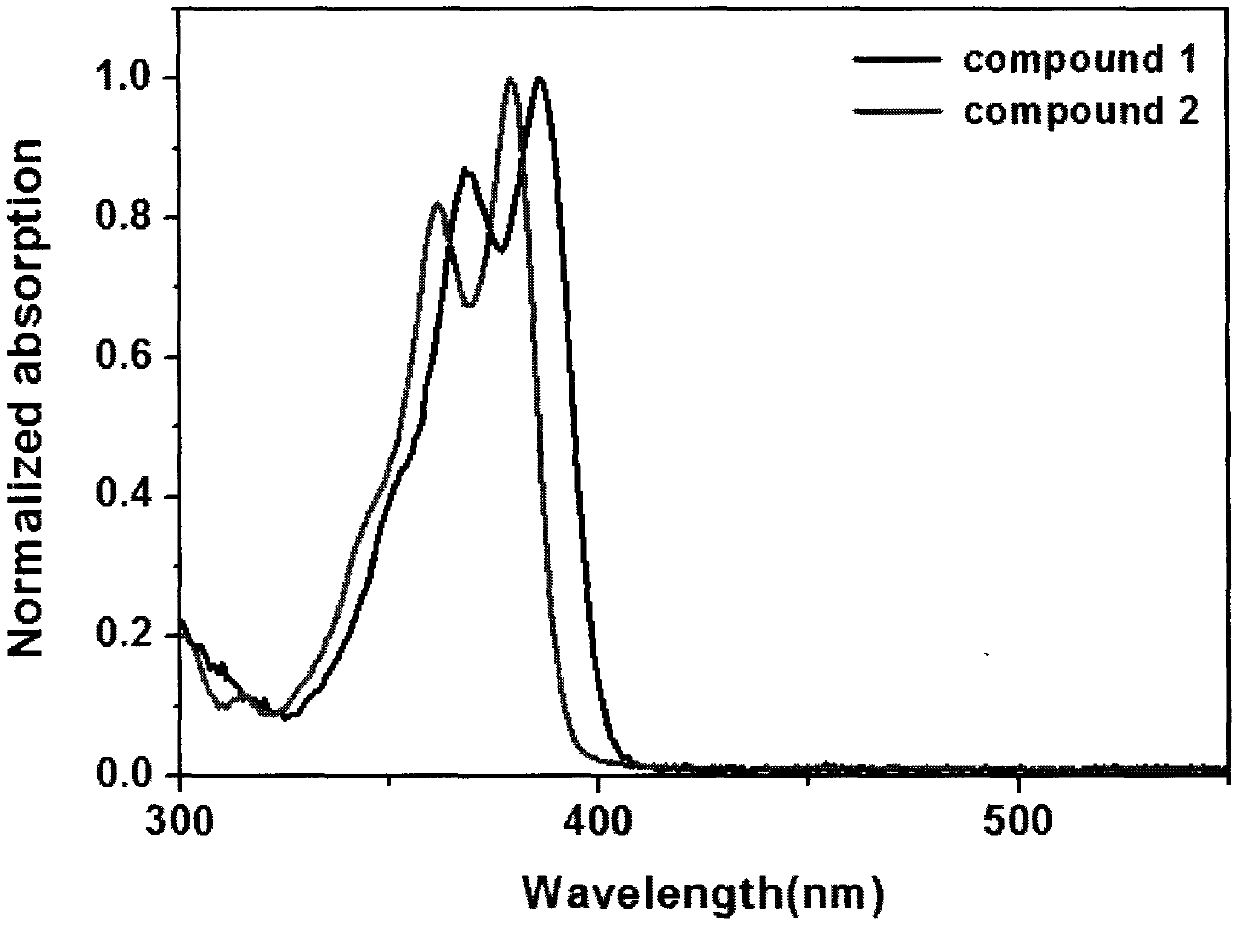
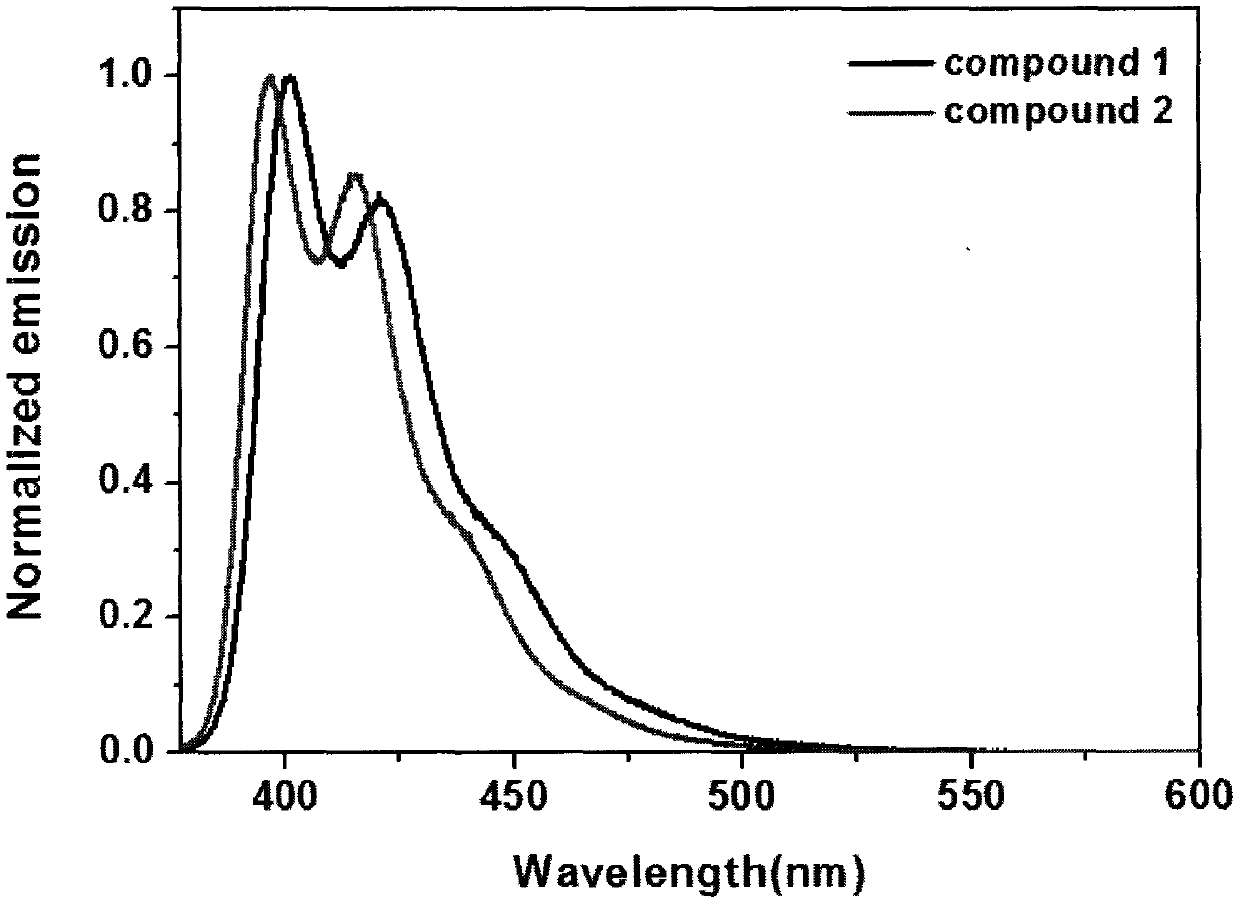
[0034] Some of the above-mentioned compounds are given examples, and the details are as follows:
Embodiment 1
[0035] Embodiment 1: the synthesis of compound 1
[0036] 1) Synthesis of Compound 4: Weigh 1,5-difluoro-2,4-dinitrobenzene (1.00equiv, 29.40mmol, 6.00g) and sodium hydroxide (2.20equiv, 64.68mmol, 2.59g), The gas was exchanged three times, protected by nitrogen, and 50 mL of dimethyl sulfoxide solution was added, followed by pyrrole (2.20 equiv, 64.68 mmol, 4487 μl), and stirred at room temperature for 12 h. After the reaction was complete, water and ethyl acetate were added to extract, the organic layers were combined, dried over anhydrous magnesium sulfate, filtered, spin-dried, and quickly separated by column chromatography to obtain the yellow solid target compound 4 (5.75 g, yield 65%).
[0037] 1 H NMR (400MHz, CDCl 3 ): δ8.47(s, 1H, Ar), 7.54(s, 1H, Ar), 6.83(t, J=2.4Hz, 4H, Ar), 6.45(t, J=2.4Hz, 4H, Ar).
[0038] 2) Synthesis of compound 5: Weigh 1,5-dipyrrole-2,4-dinitrobenzene (1.0equiv, 1.97mmol, 586mg) and dissolve it in 20mL ethanol solution, add bismuth chlor...
Embodiment 2
[0042] Embodiment 2: the synthesis of compound 2
[0043] 5) Synthesis of compound 6: Weigh 1,5-dipyrrole-2,4-diaminobenzene (1.00equiv, 2.10mmol, 500mg), ventilate 3 times, protect with nitrogen, add 10mL of tetrahydrofuran solution, in -78 Add n-butyllithium (2.10equiv, 4.41mmol, 1.76mL, 2.5mol / L in hexane), 0.68mL) at ℃, stir at -78℃ for 1h, add hexyl bromide (4.00equiv, 8.40mmol, 1.39 g), then heated at 66°C under reflux for 4 hours, after the reaction was complete, the solvent was spin-dried, extracted with water and ethyl acetate, the organic layers were combined, dried with anhydrous magnesium sulfate, filtered, spin-dried, and quickly carried out column chromatography After separation, the target compound 6 (436 mg, yield 51%) was obtained as brown-red oily liquid.
[0044] 1 H NMR (400MHz, CDCl 3 ): δ6.97(s, 1H, Ar), 6.75(t, J=2.0Hz, 4H, Ar), 6.31(t, J=2.0Hz, 4H, Ar), 5.98(s, 1H, Ar), 3.72 (br, 2H, NH 2 ), 3.12(t, J=6.8Hz, 4H, CH 2 ), 1.53-1.56 (m, 4H, CH 2 ), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com