Application of benzothiazoles and benzopyrroles in the preparation of antitumor drugs
An anti-tumor drug, benzothiazole technology, applied in the field of medicine, can solve problems such as benzothiazole and benzopyrrole compounds and their pharmaceutical compositions that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
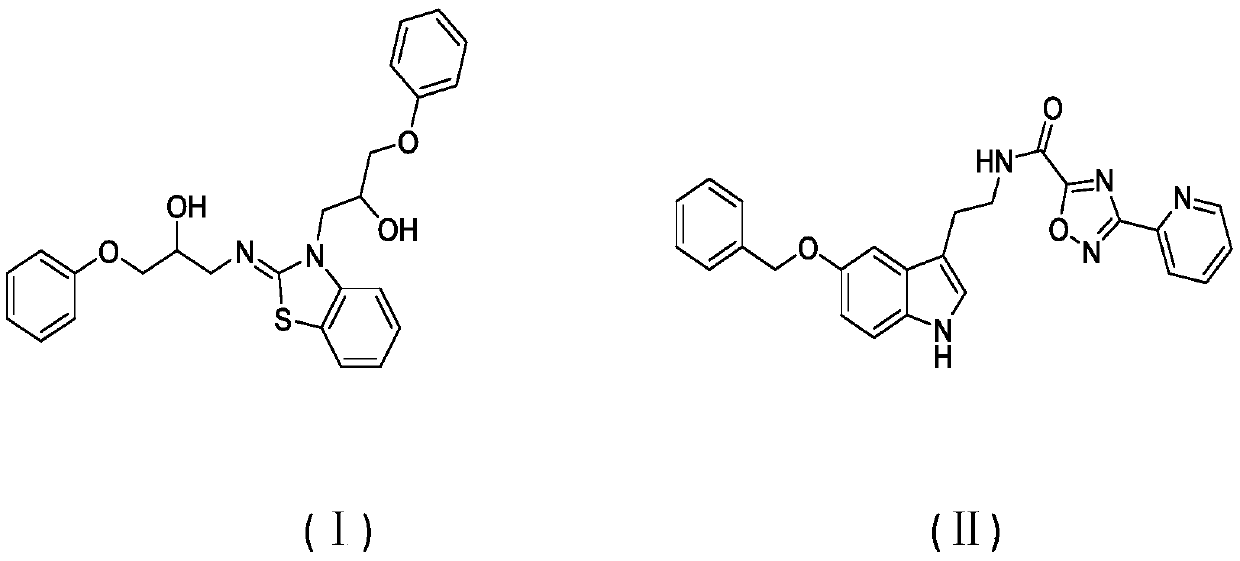
[0027] The compound of the present invention was partially purchased from IBScreen, and the corresponding numbers of compounds I and II in the library are: STOCK1S-28804; STOCK6S-58881.
[0028] The structural formulas of compounds I and II are as follows:
[0029]
Embodiment 2
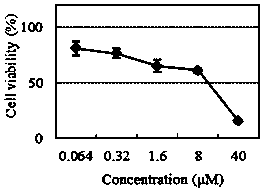
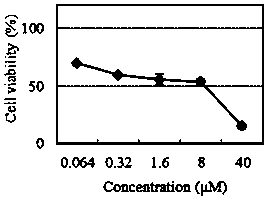
[0031] The half growth inhibitory concentration IC of the compound of the present invention on five different tumor cell lines 50 Determination of:
[0032] 1. Experimental principle and steps
[0033] MTS is an analog of MTT. The succinate dehydrogenase in the mitochondria of living cells can metabolize and reduce MTS to generate soluble formazan (Formazan) compounds, which can be directly dissolved in the culture medium. When testing, only a small amount of CellTiter Aqueous One Solution reagent is directly added to the medium of the culture plate well, incubated for 1-4h, and then read the absorbance value at 490nm with a microplate reader. The optical density OD (490nm) of the compound is directly proportional to the number of living cells. Cisplatin and paclitaxel were used as positive controls. Compound IC 50 The value is determined by calculation of the concentration effect growth curve.
[0034] Let stand for 90 minutes at room temperature to completely melt CellTiter Aq...
Embodiment 3
[0049] Compound I or II is added with 4% sulfuric acid ethanol solution, pH=4, filtered and dried to prepare sulfate compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com