Novel organic electroluminescent material based on benzopyrroledione and application thereof
A technology of diketone benzopyrrole and electroluminescent materials, which is applied in the fields of luminescent materials, organic chemistry, circuits, etc., and can solve problems such as low efficiency and short device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
[0021] Synthesis of compound 1
[0022] 2-Hydroxy-2-phenylacetic acid (10g, 0.066mol), p-phenylenediamine (3.2g, 0.03mol) and chlorobenzene solution (100mL) were added to a 250mL single-necked bottle, and the mixture was heated to 130°C under nitrogen and stirred 18 hours. After the reactant was cooled to room temperature, it was poured into distilled water (100 mL); the mixture was washed with CH 2 Cl 2 (3×30mL) extraction; the organic layer was successively washed with water (60mL), dried, and evaporated under reduced pressure to remove the solvent; the residue was recrystallized with ethanol to obtain 7.3g of white solid (yield: 65%). 1 H NMR (CDCl 3 ,400M)δ:9.8(s,2H),7.57-7.52(m,4H),7.33-7.28(m,10H),6.02(s,2H),4.93(s,2H).
[0023] Synthesis of Compound 2
[0024] Compound 1 (6.0 g, 0.016 mol) and concentrated sulfuric acid (20 mL) were added into a 100 mL one-necked flask, and the mixture was stirred overnight at room temperature under nitrogen. After the...
Embodiment 2
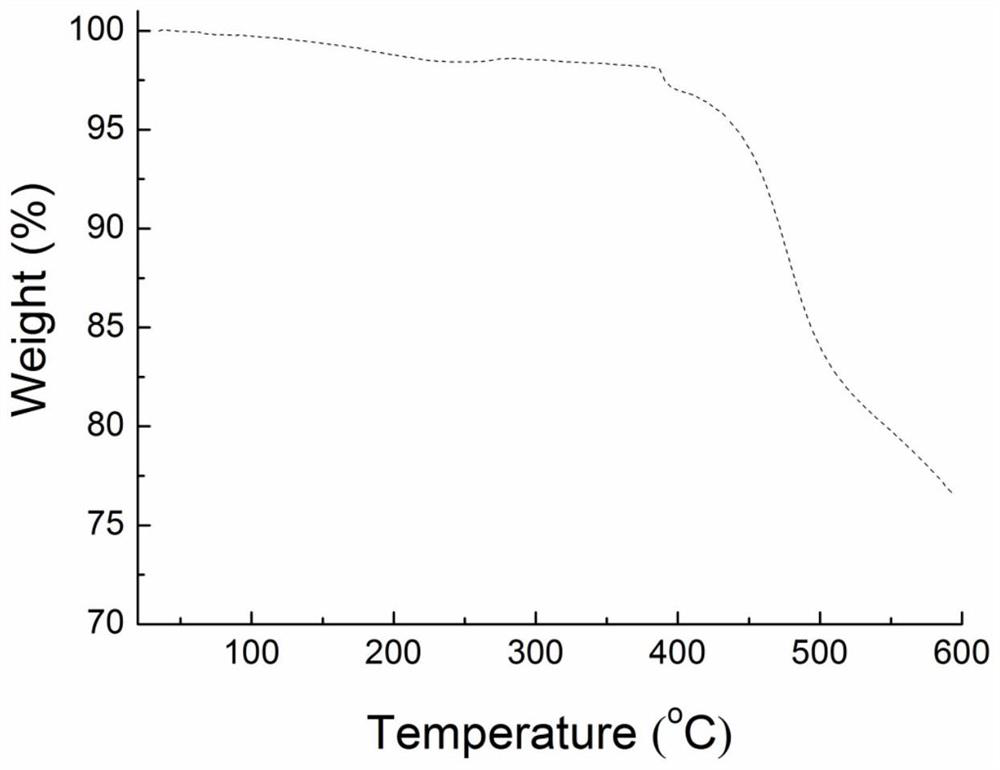
[0034] Thermal stability test of H-DPP-CN in Example 1.
[0035]Under a nitrogen atmosphere, the heating rate was 20° C. / min, and the thermal stability of the compound H-DPP-CN was tested by a thermogravimetric analyzer. Depend on figure 1 It can be seen that the thermal decomposition temperature of the compound H-DPP-CN at a weight loss of 5% is greater than 400°C, indicating that the compound has good thermal stability and can be used to prepare electroluminescent devices by vacuum deposition.
Embodiment 3
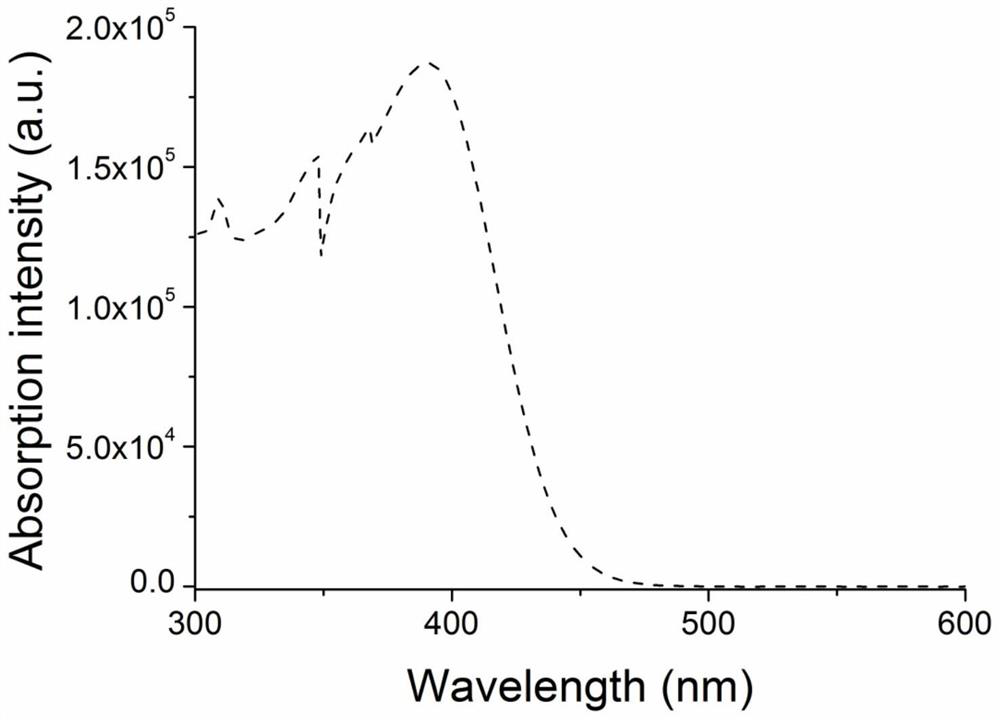
[0037] The ultraviolet-visible absorption spectrum test of the compound H-DPP-CN in Example 1.
[0038] Compound H-DPP-CN was dissolved in toluene to form 10 -5 M solution, test the UV-Vis absorption spectrum of its solution at room temperature. Depend on figure 2 It can be seen that the compound H-DPP-CN has a strong UV-Vis absorption peak in the range of 300-450nm, which is mainly attributed to the transition absorption of π-π* in the molecule.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com